Determination of Corrosion Resistance of Coating Films by Volume Test
When determining the degree of corrosion of metals, both gravimetric and volumetric methods can be used. In the former case, the corrosion product is collected and weighed, and in the second case, the volume of hydrogen gas evolved or oxygen absorbed is measured. The volume test method has great accuracy; with this instrument, the corrosion process at different times can be easily retrieved.
The volume test method has not been widely used for testing paint coatings, although this method has proved to be suitable for dry testing coatings on light metals and their alloys (aluminum-magnesium alloys).
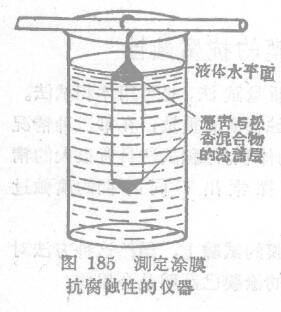
Determination of corrosion resistance of coating film by HNNJIK method
Buck once proposed a method for measuring the anti-corrosion properties of paint coatings on iron surfaces; Based on the determination of the generated iron ions. In this case, potassium persulfate acts not only as a corrosive electrolyte, but also as an oxidizing agent that converts iron into the trivalent form.
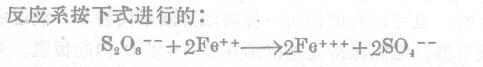
Ammonium thiocyanate is an active corrosive agent, and it also acts as an indicator when it forms iron thiocyanate with trivalent iron. The concentration of iron thiocyanate measured by a light Colorimeter or an ordinary Colorimeter is the standard for judging the corrosion resistance of the tested coating film. When preparing the electrolyte, 13.5 grams of potassium persulfate and 15 grams of ammonium thiocyanate were dissolved in 1.1 liter of water.
The rod coated with the sample is coated with a mixture of hot-melt astringent green and rosin (1:1) from both ends (at a distance of 1 cm from the rod end); after that, the rod is immersed in a volume of 150 ml In the beaker filled with the electrolyte, the water was slowly brought up to the part covered with the rosin mixture. After a certain period of time, such as a day and night, take out the rod, pour the colored solution into the side ditch of the light Colorimeter, and measure the concentration of iron according to the inclination of the galvanometer pointer. At the same time, the more heavily colored the solution is, the worse the corrosion resistance of the paint film will be. A necessary condition for carrying out this test is that all films being compared must be of the same thickness.
A comparison table of the relationship between the readings of the galvanometer and the concentration of iron in the solution has been compiled, which can be used to quantitatively check the corrosion resistance of the coating film. This method can also be successfully used in the test of the water permeability of the coating film. (Related instrument: conductivity meter)
Buck once proposed a rapid method for measuring the corrosion resistance of paint coatings on light metals, which is based on replacing copper in 0.02H.copper sulfate solution with basic components in light metal alloys—aluminum and magnesium;
The change in copper concentration can be measured by electrolyte.
This method is more complicated, which is its disadvantage.