A PH METER IS AN INSTRUMENT USED TO MEASURE THE PH of a solution by measuring the concentration of hydrogen ions in the solution. It is usually composed of a glass electrode and a reference electrode, which reflects the acid-base characteristics of the solution through the potential difference, and is widely used in laboratory and industrial production to ensure the accuracy of product quality and process control.PROPER USE AND MAINTENANCE OF A PH METER IS ESSENTIAL TO ENSURE ACCURATE AND RELIABLE ACIDITY MEASUREMENTS AND TO EXTEND THE LIFE OF THE INSTRUMENT WHILE MAXIMIZING PRODUCTIVITY.
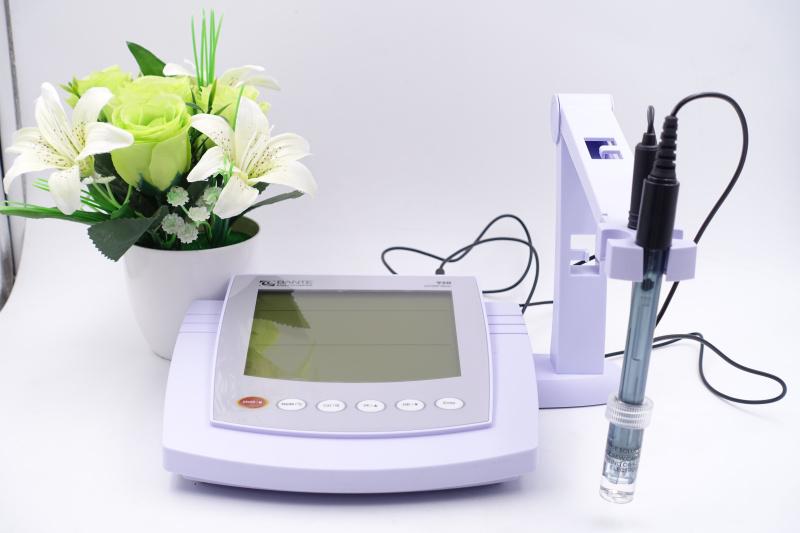
Daily use and maintenance
Glass electrodes
Unused glass electrodes should be stored in a cool, dry place to prevent contamination and possible electrical leakage or corrosion. Before use, the glass membrane section should be fully immersed in distilled water or dilute hydrochloric acid for a recommended period of time (usually overnight) to ensure adequate hydration.
Double-check the electrodes for cracks or spots before measuring. Make sure that the internal solution fills most of the cavity and that the auxiliary electrodes are properly immersed as well.
Before immersing in the solution being tested, rinse the electrode with distilled water to avoid contaminating the sample. Special care is taken when handling weak or trace solutions to prevent mechanical damage to the glass membrane.
Reference electrode and salt bridge
Keep the reference electrode chamber filled with a saturated potassium chloride solution to maintain constant contact with the solution. For silver-silver chloride electrodes, add silver chloride crystals at regular intervals. Make sure that the salt bridge remains unobstructed, especially in solutions that are prone to precipitation or turbidity.
Electronic components
Understand the fundamentals and follow the manufacturer's instructions to avoid unnecessary damage. Ensure that the working environment meets the specified conditions for temperature, humidity and stability to prevent corrosion or failure.
calibration
Use standard buffer solution pairs regularlyPHThe gauge is calibrated to verify the accuracy and repeatability of the measurement results. Adjust the position and settings of the instrument as needed to ensure reliable performance over different pH ranges.Before measuring the sample, calibrate the standard buffer solution that is close to the expected range of the sample. For precision measurements, the test is repeated to confirm consistency within the allowable tolerance.
Operational precautions
Avoid splashing the solution on the instrument. Keep electrodes and contacts clean and dry to prevent corrosion and ensure good electrical contact.Regularly inspect appearances, fasteners, and operating controls. Store the instrument safely after use, making sure all settings are correct and readings are within the expected range.Proper use and maintenance of an acidity meter requires careful care of the electrodes, regular calibration, and adherence to operating guidelines. By following these practices, users can enhance the accuracy and reliability of acidity measurements while extending the life of the instrument.
