The acidity meter is mainly used to measure the electromotive force to detect the pH value of the aqueous solution, also known as the pH meter. It can be used in the measurement of electrode potential or other aspects. Commonly used pH meters in laboratories include pH-25 (Lei Magnetic 25), pHS-2, pHS-3E, pHS-3B, etc. Its operation and application are explained in detail as follows.
Measuring principle
The acidity meter measures the pH value by inserting a pair of working electrodes into the solution to be tested (one is a reference electrode with a known and constant electrode potential, and the other is an electrode whose potential changes with the ion concentration of the solution to be tested. Indicating electrode) constitutes a primary battery, and connected to a precision potentiometer, the electromotive force of the battery can be measured. Since the pH value of the solution to be tested is different, the electromotive force generated is also different. Therefore, the pH value of the solution to be tested can be measured by measuring the electromotive force of the solution with an acidity meter.
In order to save the calculation procedure of converting the electromotive force into a pH value, the measured electromotive force of the battery is usually expressed directly on the electric dial with a pH scale value. At the same time, the instrument is also equipped with a positioning regulator. When measuring, use the pH standard buffer solution first, and make the pointer on the instrument just point to the pH value of the standard solution by positioning the regulator. In this way, when measuring an unknown solution, the pointer directly indicates the pH value of the solution to be tested. Usually the former step is called calibration, and the latter step is called measurement.
The E-201-C9 composite electrode used in the pHS-3B instrument is composed of a pH glass electrode and a silver-silver chloride electrode. The glass electrode is used as a measuring electrode, and the silver-silver chloride electrode is used as a reference electrode. When the measured solution When the hydrogen ion concentration changes, the electromotive force between the glass electrode and the silver-silver chloride electrode also changes, and the electromotive force change relationship conforms to the following formula:

Instrument structure
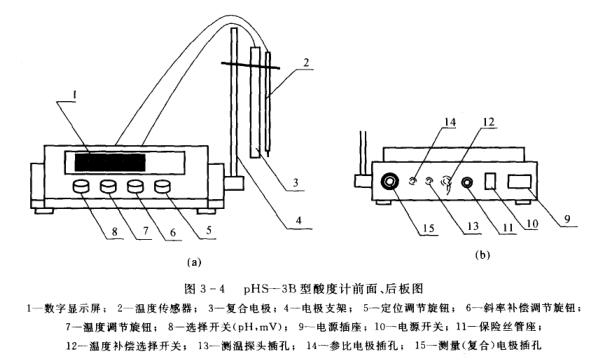
There are many types and models of acidity meters, but they are all composed of three parts: a reference electrode (commonly used as a calomel electrode), an indicating electrode (commonly used as a glass electrode) and a precision potentiometer. The panel diagram of the pHS-3B acidity meter is shown in Figure 3-4.
Steps
1. Preparation before starting up
(1) Screw the electrode holder into the electrode holder socket, and adjust the electrode clamp to a proper position.
(2) Clamp the composite electrode and T811 temperature sensor on the electrode clip, and pull off the electrode sleeve at the front end of the electrode.
(3) Clean the electrodes with distilled water. After washing, wash again with the solution to be tested.
2. Boot
(1) Plug the power cord into the power outlet. Press the power switch, after the power is turned on, preheat for 30 minutes, and then carry out calibration.
(2) Use of pH automatic temperature compensation and manual temperature compensation:
1) As long as the switch 12 on the rear panel is placed in the automatic position, the instrument can be in the state of automatic pH value temperature compensation, and manual temperature compensation does not work at this time.
2) The method of using manual temperature compensation: remove the temperature sensor, and set the switch 12 on the rear panel to the manual position. Set the "selection" switch 8 of the instrument to the "°C" of the "temperature regulator" to adjust, so that the digital display value is the same as the thermometer display value in the measured solution, and the instrument also sends the temperature signal to pH-, and the hybrid circuit performs calculations. So as to achieve the purpose of manual temperature compensation.
(3) Solution temperature measurement method: Set the "selection" switch of the instrument to "℃", and the digital display value is the temperature value measured by the temperature sensor.
 3. Calibration
3. Calibration
The instrument must be calibrated before use. Generally speaking, when the instrument is in continuous use, it should be calibrated once a day.
(1) Unplug the short-circuit plug at the measuring electrode jack 15; insert the composite electrode 3 and the T811 temperature sensor 2 at the measuring electrode jack.
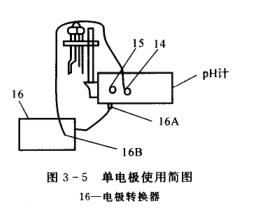
(2) If the composite electrode is not used, insert the electrode converter plug 16A at the measuring electrode jack 15; insert the glass electrode plug into the converter socket 16B; connect the reference electrode to the reference electrode jack 14 (see figure 3-5).
(3) Turn the "selection" switch knob 8 to the pH gear.
(4) Measure the temperature of the solution first, and set the "selection" switch to "°C". The digital display value is the temperature of the solution.
(5) Turn the slope adjustment knob 6 clockwise to the end (that is, to the 100% position).
(6) Insert the cleaned electrode into the buffer solution with pH=6.86.
(7) Adjust the positioning knob so that the readings displayed by the instrument are consistent with the pH value of the buffer solution at the current temperature (for example, when the temperature is 10°C with mixed phosphate, pH=6.92) (8) Clean the electrode with distilled water, and then insert pH = 4.00 (or pH rate knob to make the instrument display read the same as the pH value at the current temperature in the buffer.
(9) Repeat (7)~(9) until there is no need to adjust the two knobs of positioning or slope.
(10) The instrument is calibrated. Calibration by manual temperature compensation can refer to item (2) of operation step two.
Note: After calibration, the positioning adjustment knob and slope adjustment knob should not be changed.
The calibrated buffer solution should be pH=6.86 for the first time, and should be close to the value of the measured solution for the second time. If the measured solution is acidic, the buffer solution should be pH=4.00; if the measured solution is alkaline, Choose a buffer solution with pH=9.18. Under normal circumstances, the instrument does not need to be re-calibrated within 24 hours.
4. Measuring pH
When measuring the pH value of the solution, the electrode should be cleaned with distilled water first, and then the electrode should be cleaned with the solution to be tested, then the electrode should be inserted into the solution to be tested, and the beaker should be shaken to make the solution uniform and then read the pH value of the solution.
5. Measure the electrode potential value
(1)将离子选择电极或金属电极和甘汞电极夹在电极架上。
(2)用蒸馏水清洗电极头部,用被测溶液清洁一次。
(3)把电极转换器的插头16A插入仪器后部的测量电极插座内;把离子电极的插头插入转换器的插座16B内。
(4)把甘汞电极接入仪器后部的参比电极接口上。
(5)把两种电极插在被测溶液内,将溶液搅拌均匀后,即可在显示屏上读出该离子的选择电极的电极电势值,还可自动显示正负极性。
(6)如果被测信号超出仪器的测量范围,或测量端开路时,显示屏会不亮,作超载报警。
仪器维护
pH计具有很高的输人阻抗,使用环境经常接触化学药品,为保证仪器正常使用,所以更需要合理维护。
(1)仪器的输人端(测量电极插座15)需要保持干燥清洁。仪器不用时,将短路插头插人插座,防止灰尘及水气进入。在环境温度较高的场所使用时,应把电极插头用干净纱布擦干。
(2)测量时,电极的引入导线应保持静止,否则会引起测量不稳定。
(3)仪器采用了M0S集成电路,因此,在检修时应保证电烙铁接地良好。
(4)用缓冲溶液标定仪器时,要保证缓冲溶.液的可靠性,不能配错缓冲溶液,否则测量结果可能产生误差。
缓冲溶液用完后可按下列方法自行配制:
1)pH=4.00溶液:用GR邻苯二甲酸氢钾10.21g,溶解于1000mL的高纯去离子水中。
2)pH=6.86溶液:用GR磷酸二氢钾3.4g,GR磷酸氢二钠3.55g,溶解于1000mL的高纯去离子水中。
3)pH=9.18溶液:用GR硼砂3.18g,溶解于1000mL的高纯去离子水中。
(5)测温传感器采用pt100线型热敏电阻,使用寿命长,但切勿敲击或摔伤,如遇到温度传感器损坏,可再向厂方购买。在温度传感器损坏情况下,可使用手动温度补偿进行测量。
电极使用维护的注意事项
(1)电极在测量前需要用已知pH值的标准缓冲溶液进行定位校准,其值愈接近被测值愈好。
(2)取下电极套后,应避免电极的敏感玻璃泡与硬物接触,因为任何破损或擦毛都可能使电极失效。
(3) After the measurement, put the electrode protective sleeve on in time, and put a small amount of replenishing liquid in the sleeve to keep the electrode bulb moist. Do not soak in distilled water.
(4) The supplementary solution for the external reference of the composite electrode is 3m01/L potassium chloride solution, and the supplementary solution can be added from the small hole at the top of the electrode.
(5) The lead-out ends of the electrodes need to be kept clean and dry to absolutely prevent short-circuiting at both ends of the output, otherwise it will lead to measurement inaccuracy or failure.
(6) The electrode should be matched with a acidity meter with high input impedance (≥10 12 Ω) so as to maintain good characteristics.
(7) The electrode should avoid long-term immersion in distilled water, protein solution and acidic fluoride solution.
(8) Avoid contacting the electrodes with silicone oil.
(9) After the electrode has been used for a long time, if the slope is found to be slightly reduced, the lower end of the electrode can be soaked in 4% HF (hydrofluoric acid) for 3~5s, washed with distilled water, and then soaked in 0.lm01/L hydrochloric acid solution Soak it in water to rejuvenate it.
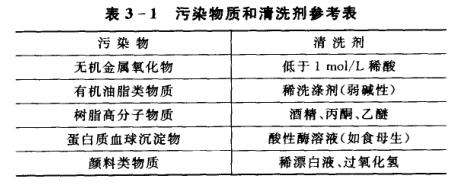
(10) If the measured solution contains substances that are easy to contaminate the sensitive bulb or block the liquid junction and passivate the electrode, the slope will decrease and the displayed reading will be inaccurate. If this phenomenon occurs, it should be cleaned with an appropriate solution according to the nature of the polluting substance to renew the electrode.
Note: When choosing a cleaning agent, do not use carbon tetrachloride, trichlorethylene, tetrahydrofuran and other cleaning waves that can dissolve complex carbonic acid resins, because the electrode casing is made of basic carbonic acid resins, which can easily contaminate sensitive glass after dissolution. The ball bubbles, thereby rendering the electrode useless. Also can't use compound electrode to measure above-mentioned solution.
The above are some structure, operation application, precautions and maintenance instructions of the author on the acidity meter (PH meter), hoping to provide some references for relevant laboratory personnel.
 3. Calibration
3. Calibration