The commonly used methods for measuring the conductivity of electrolyte solutions in the laboratory include the balanced bridge method and the unbalanced bridge method. The former commonly used instrument is Wheatstone bridge, and the latter mostly uses domestic DDS-l IA conductivity meter or DDS-1 1 type conductivity meter.
DDS-11 Conductivity Meter or DDS-1 IA Conductivity Meter is an instrument for measuring liquid conductivity. These two instruments are direct-reading, with a wide measurement range and the change of value. Easy to operate. If it is equipped with an automatic balance Recorder, it can automatically record the conductance value or conductivity. The following is a brief introduction to the DDS-1 IA conductivity meter.
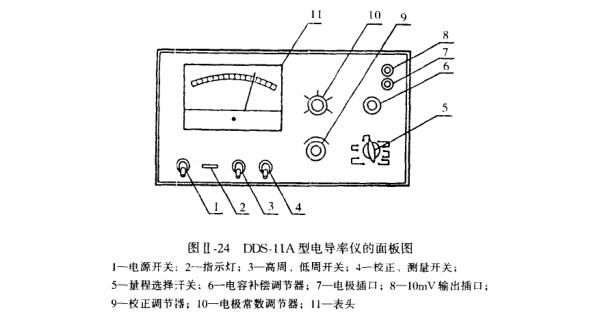
1. Appearance of the instrument
The appearance of the instrument is shown in Figure I1-24.
2. Measuring principle

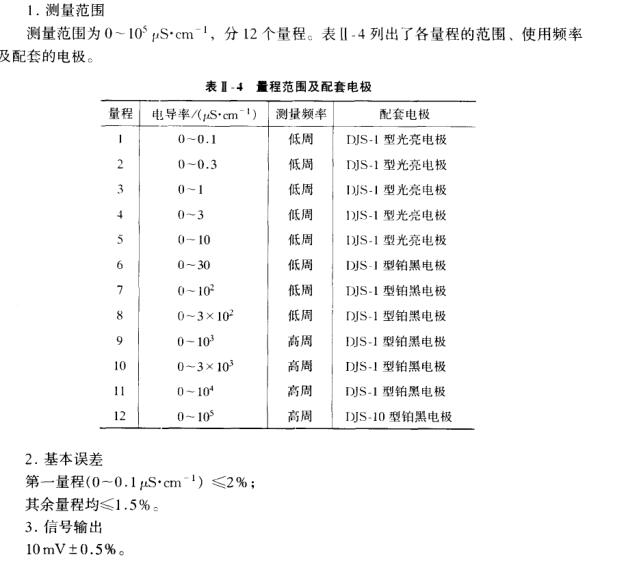
3. Measuring range

4. How to use

5. Matters needing attention
(1) The lead wire of the electrode should not be wet, otherwise the measurement will be inaccurate.
(2) After the high-purity water is filled into the container, it should be measured quickly, otherwise the CO 2 in the air will dissolve into the water, which will cause the conductivity to increase rapidly.
(3) The container containing the solution to be tested must be free from ion contamination.
(4) After each sample is measured, do not directly rinse the platinum black on the electrode with distilled water; also do not rub the platinum black, so as not to fall off the platinum black and cause changes in the electrode constant. It can be rinsed with the solution to be tested 3 times before the determination.