1. Electrochemical corrosion
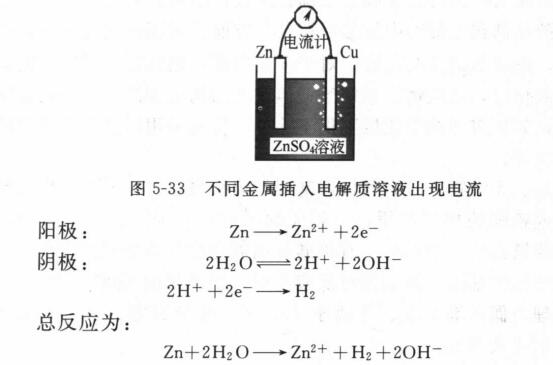
If a wire is connected to two different metal plates immersed in a certain electrolyte, a current flows and an electrochemical reaction occurs, and this structure constitutes a primary battery. If it is composed of zinc plate and copper plate, it will be found that the zinc plate will be consumed gradually, and bubbles will appear on the copper plate (Figure 5-33). The zinc plate acts as the anode of the battery and the copper plate acts as the cathode, and an electrochemical reaction takes place between the two metal plates.
By studying different metal combinations, the electromotive series of metals can be arranged, such as Mg>A1>Zn>Fe>Sn>Cu. When two metals are connected in the electrolyte, the metal at the front is the anode, and the metal at the back is the cathode. For example, Zn-Fe is paired, and Zn is the anode; while Fe-Cu is paired, Fe is the anode. The former Zn is corroded, and the latter Fe is corroded. The reactivity of various metals can also be judged according to their standard electrode potential values (Table 5-7). The higher the negative value of the standard potential, the easier it is for the metal to be oxidized into ions; on the contrary, the higher the positive value of the standard potential, it indicates The metal is less likely to be oxidized.
There are many types of steel, all of which are alloys of iron and carbon with other metals. Various steels have different sensitivities to corrosion due to their different compositions and the presence of mechanical strain on steel parts. The heterogeneous composition in the structure can easily cause electrochemical reactions, which can lead to the corrosion of iron. For example, cold-rolled steel is more susceptible to corrosion than hot-rolled steel, and anodic and cathodic regions can also form near the point of impact on the impacted steel plate.
2. Influencing factors
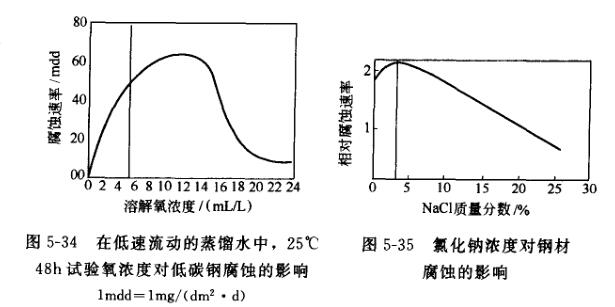
(1) Dissolved oxygen As shown in Figure 5-34, as the oxygen concentration in the solution increases, the corrosion rate also increases, but then decreases after reaching the maximum value.
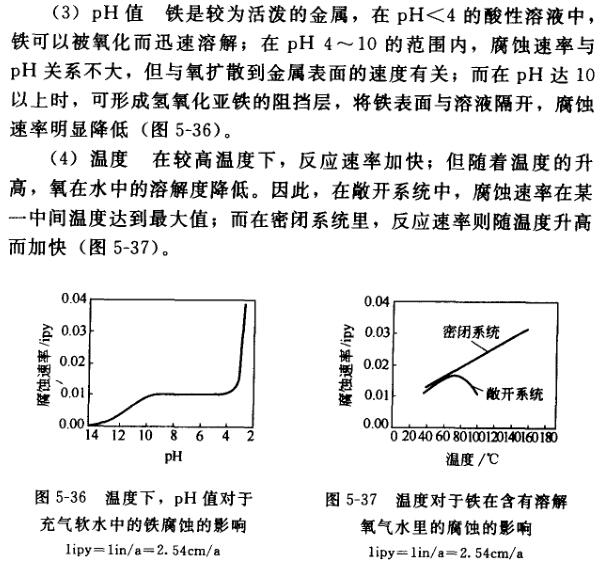
(2) The formation of salt concentration electrochemical corrosion requires a complete circuit, so the presence of salt will undoubtedly increase the conductivity of water, thereby accelerating corrosion. At the same time, iron chloride or sulfate is more soluble than ferrous hydroxide, thus accelerating the diffusion rate of iron ions into the solution, resulting in an increase in corrosion rate. It has also been suggested that sodium ions can react with the thin layer of iron oxide on steel to form sodium hydroxide, which will also accelerate corrosion. Figure 5-35 shows the relationship between sodium chloride in water and the corrosion rate of steel. The dashed vertical line is the salt concentration in seawater. Above this concentration, the corrosion rate decreases with the increase of salt concentration. This is because the solubility of oxygen in the salt solution decreases with the increase of salt concentration.