1 Principle of
When a beam of monochromatic light passes through a certain solution, part of the light energy is absorbed, and the intensity of the light is weakened. The relationship between light intensity and solution concentration can be expressed by the Braun-Beer law:
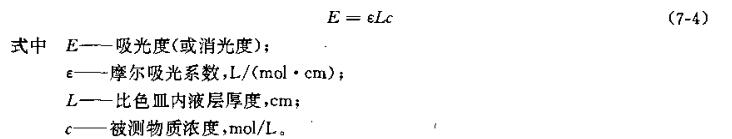
According to Lambert-Beer's law, when the thickness (L) of the liquid layer of the measured solution is constant, the concentration (c) of the substance in the solution is proportional to the absorbance (E). That is, the greater the concentration, the darker the color, the greater the degree of light absorption, and the smaller the intensity of light at this time. The light intensity is converted into electric current through the photocell, and the absorbance of the measured substance is indicated from the magnitude of the electric current, and then compared with the standard solution, the concentration of the measured solution can be known.
2. Construction and use of photoelectric Colorimeter
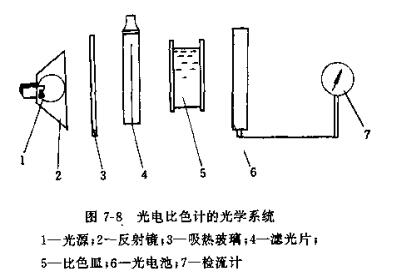
(1) Optical system
The optical system of the photoelectric Colorimeter is shown in Figure 7-8. The light emitted from the bulb 1 is reflected by the reflector 2, passes through the heat-absorbing glass 3 and the filter 4, then passes through the solution in the cuvette 5, and reaches the photocell 6. The photocurrent is measured by galvanometer 7.
Light source: Colorimeters generally use tungsten filament bulbs as the light source, and the voltage of the light source needs to be stable. Usually, a battery or a regulated power supply provides a voltage of 6~12V.
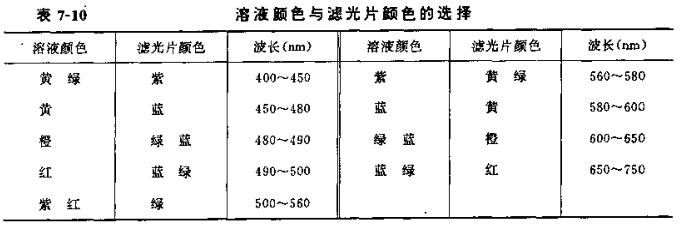
Light crossing film: light crossing film is a kind of colored glass, white light can be approximated as monochromatic light after passing through it. There are 3 to 9 colors of commonly used light crossing film. According to the color of the solution to be measured, the filter is selected according to the principle of light complementarity. For example, potassium percyanate solution is purple-red, and it chooses to absorb the most green light, so a green filter should be selected. Table 7-l0 lists the choice of the color of the solution and the color of the filter.
Cuvette: The cuvette is also called the cuvette, which is made of transparent, colorless and corrosion-resistant flat optical glass or quartz. Generally, Colorimeters are equipped with sets of cuvettes with different optical path lengths (0.5, 1, 2, 3, 5 cm, etc.). In order to improve the accuracy of the measurement, it is necessary to select the colorimetric cuvette before the measurement. The method is as follows: inject distilled water into several cuvettes of the same specification, and use any one of the cuvettes as a blank, and test the color at a wavelength of 440nm ( Measure their light transmittance on the blue) respectively, and choose the light transmittance with a difference of less than 0.5.
Selenium photocells are commonly used in photocell Colorimeters. It is composed of translucent cadmium oxide film, semiconductor sun and iron (or aluminum foil) base film, as shown in Figure 7-9.
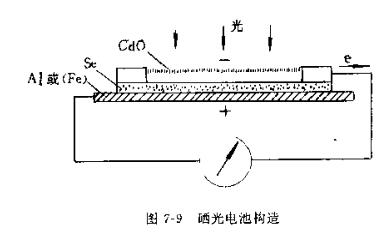
When the light shines on the semiconductor selenium through the semi-transparent film, electrons will escape, and due to the unidirectional conductivity of the semiconductor, the electrons will flow from the selenium to the metal base film. The measurable spectral region of the solar cell is the visible part, ranging from 380 to 750nm, among which it is most sensitive to light with a wavelength of 560nm. Therefore, it is not suitable for infrared light and ultraviolet light, and the special photocell is sensitive to infrared light and ultraviolet light.
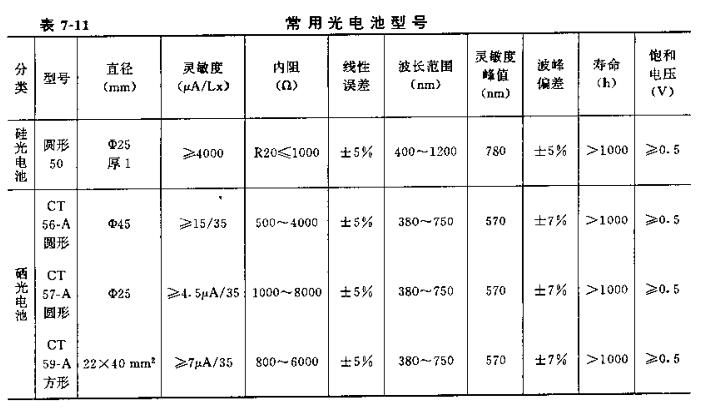
See Table 7-11 for the specifications and performances of several domestic photovoltaic cells.
Photoelectric tube: Its structure is that the bright and positive poles are installed in the vacuum tube, and the surface of the cathode is coated with photoelectric emission materials. After being irradiated by light, electrons are emitted, and after a voltage can be applied to the two poles, the anode absorbs electrons to form an electron flow, and the electron flow changes with the light intensity. Weak light can be measured with a photocell. The domestically produced GD-5 photoelectric tube is coated with antimony zinc material on its bright pole surface, which is sensitive to ultraviolet light, and the measurable wavelength range is 200~625nm; GD-6 photoelectric tube, its bright pole surface is coated with silver chain material , Sensitive to infrared light, the measurable wavelength range is 625~1000nm. The sensitivity of the photomultiplier tube is higher, and this kind of phototube can convert extremely weak light into a large current.
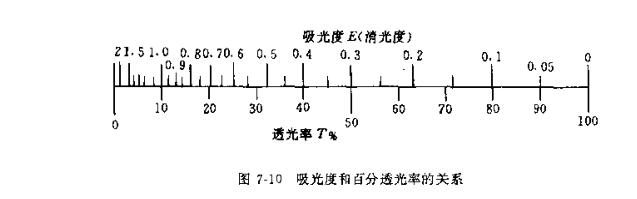
Galvanometer: The galvanometer commonly used in photoelectric Colorimeters is a compound spot reflection galvanometer with a sensitivity of 10 -8 ~ 10 -9 A /mm. There are two kinds of marking scales, one is the light transmittance r% expressed as a percentage, and the other is the absorbance i (or extinction). The negative logarithmic relationship between the two is shown in Figure 7-10.
(2) Operation
Take the 581_G photoelectric Colorimeter as an example. Place the instrument on a stable workbench, turn the selector switch and the thickness knob to "0", and then connect the power cord. The power supply voltage should meet the requirements of the instrument, the AC power socket should have a ground terminal, and the resistance to ground should be less than 10Ω.
Select the filter and trace it into the drawing hole of the light transition film. Put the cuvette containing the blank solution and the solution to be tested into the groove of the sliding plate, and at the same time, use the light-shielding differential to cover the cuvette.
Zero adjustment of the galvanometer: Turn the selector switch to "1", turn the zero point adjustment knob, so that the light spot of the galvanometer is exactly on the "0" scale of the light transmittance.
Calibration of the blank solution: put the blank solution in the optical path, turn the selector switch to 2", adjust the group adjustment knob, so that the light spot of the galvanometer is close to the light transmittance "100", and then adjust it with the fine adjustment knob until the detection The flow meter light spot is exactly on "100".
Measurement operation: gently push the cuvette containing the solution to be tested into the optical path, and read directly on the reading scale. The upper scale is transmittance (T), and the lower scale is absorbance (E).
After the measurement, turn the selector switch to the "0" position, turn the coarse and fine adjustment knobs to the "0" position, and unplug the power bracket.
(3) Precautions
The instrument should be used in normal temperature, dry, non-corrosive gas environment.
The light source cannot be switched on without the filter. If you do not want to measure during the measurement process, you can turn the selector switch to "1" so that the photocell is not exposed to light.
Pay attention to protect the light-transmitting surface of the cuvette, and do not touch the light-transmitting surface with your hands. If there is dirt, gently wipe it with lens cleaning paper. After use, the cuvette should be cleaned in time, first with plain water, then with distilled water, if necessary, with dilute hydrochloric acid, dilute nitric acid and appropriate solvents, do not use detergents, strong oxidants (such as K2 Cr 2 0 7 washing liquid) and alkaline washing.
The photocell needs to be replaced after the service life of the photocell is exceeded or the photocell fails due to accidental reasons. Since the sensitivity of each photocell is different, it is necessary to redo the working curve after replacement.
To move the Colorimeter, first pull the switch to the "0" position (the galvanometer is short-circuited at this time).
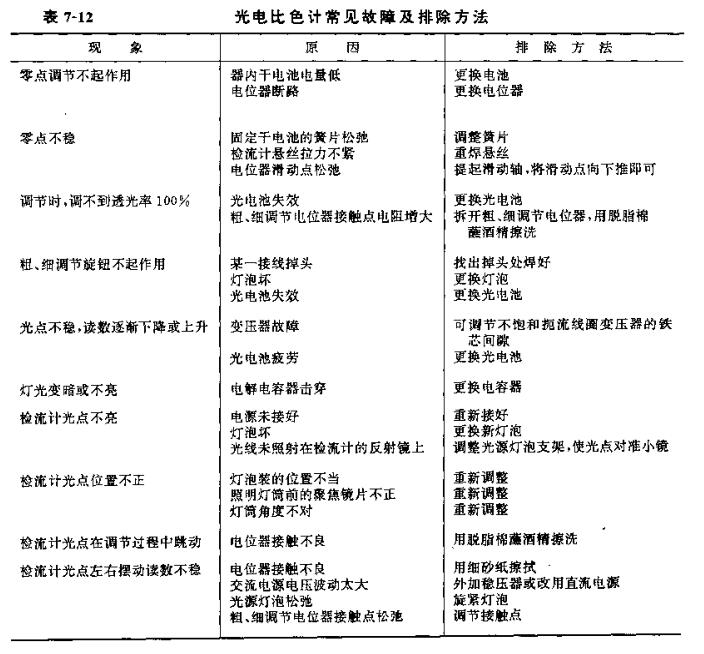
(4) Common faults and troubleshooting
See Table 7-l2 for the common faults, fault causes and troubleshooting methods of the photoelectric Colorimeter.