Gas chromatography is a new separation and analysis technique developed rapidly in the past 20 years. It has the characteristics of high separation efficiency, fast analysis speed, and less sample consumption. It has been widely used to solve analytical problems in industrial production, scientific research, and production control in petroleum, chemical, organic synthesis, medical and health, and food industries.
Fundamental
Gas chromatography is a physical and chemical analysis method, which is to separate the components of the analyzed mixture through a chromatographic column and measure it with an appropriate Detector. The components analyzed in chromatography are distributed in two immiscible phases, one of which is fixed, called the stationary phase; the other moves relatively along the stationary phase, called the mobile phase. The mobile phase of gas chromatography is a gas. Due to the differences in the physical properties of the components in the analyzed mixture, the distribution (or solubility) in the two phases is also different, and this characteristic is expressed by the distribution coefficient:
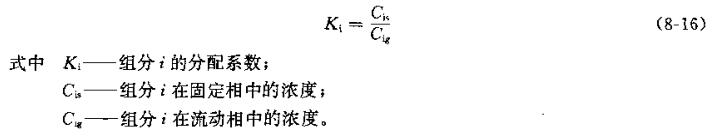
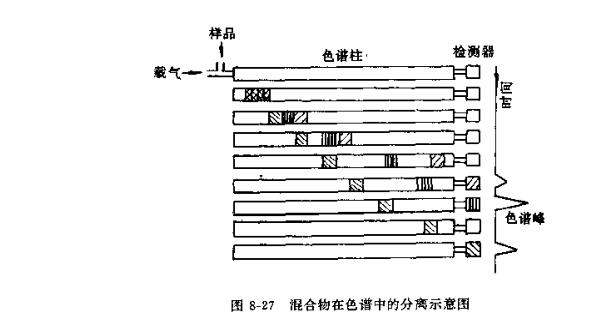
It can be seen from the formula that components with a large distribution coefficient are easily adsorbed or dissolved by the stationary phase, and stay in the stationary phase for a long time; on the contrary, components with a small distribution coefficient are poorly adsorbed by the stationary phase or have low solubility, and stay in the stationary phase for a long time. short residence time. Therefore, after repeated distribution in the column, each component is separated, as shown in Figure 8-27.
The stationary phase can be a solid with a certain surface solid activity, which can adsorb gas components; the stationary phase can also be a liquid with a high boiling point, called a stationary liquid, coated on an emotional solid substance (called a support), and the analyzed The components are dissolved in the fixative and separated due to differences in solubility.
