The use of photoelectric Colorimeters to determine a certain component is a more commonly used physical and chemical analysis method. Many substance solutions have color, and the higher the degree of the solution is, the darker the color will be. Therefore, the colorimetric analysis method can be used to determine the content of colored substances in the solution. Colorimetric classification has the advantages of sensitivity, accuracy and speed. Now take the 581-G photoelectric Colorimeter as an example to introduce its structure, principle, use and maintenance, so as to achieve the purpose of analogy.
1. Structure and principle
The principle of the photoelectric Colorimeter is to let the light beam of a certain wavelength reach the colored solution, so that the part of the light that passes through the solution is projected onto the photocell, and the intensity of the photocurrent generated by the photocell reflects the concentration of the solution. In order to achieve the purpose of photoelectric colorimetric determination. The domestic 581-G photoelectric Colorimeter is mainly composed of the following parts; light source, condenser mirror, light crossing film, solar cell, cuvette, galvanometer, etc., as shown in Figure 1.
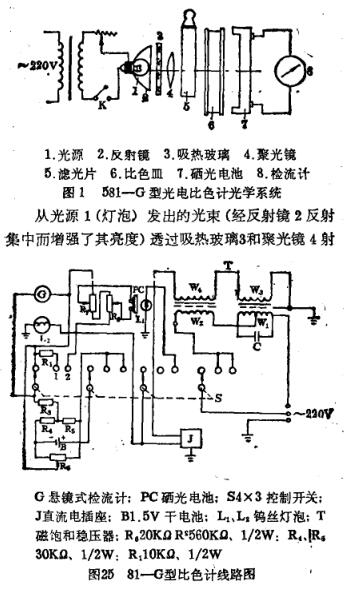
Reach the transition light sheet 5, after the polychromatic light crosses other unnecessary monochromatic light through the light transition sheet, only the monochromatic light with the same color as the transition light sheet passes through the transition light sheet and shoots into the colored solution in the cuvette 6. After the monochromatic light reaches the colored solution, a small part is reflected back, a part is absorbed by the solution, and a part passes through the colored solution. The transmitted half light hits the conductor solar cell 7, and the photo cell generates current after being lasered, and the value is indicated by the hanging mirror type galvanometer 8. The upper line of the galvanometer scale is the light transmittance 1~100, and the lower line is the logarithmic scale. Absorbance A or extinction E can be read directly from the galvanometer. The concentration of the solution can be obtained by comparing with the standard curve.
2. Electronic circuit
The electronic circuit of the 581-G photoelectric Colorimeter is shown in Figure 2
1. Power part
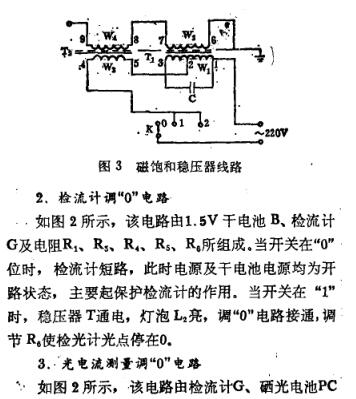
It is composed of a magnetic saturation voltage stabilizer T, a capacitor C, a direct current socket J, a light bulb L2 and an excitation light bulb L,. Use 220V 50-cycle AC or 6V DC as the power supply. The circuit of the magnetic saturation voltage stabilizer is shown in Figure 3. The primary W of the saturated coil and the capacitor C form a sugar vibration circuit, T is a saturated iron core, and Ta is an unsaturated iron core. When the saturated iron core reaches the hugging point after the voltage rises to a certain value. In his and the primary W of the line country, an additional coil is added by the base method, so that the secondary output voltage is basically stable after the magnetic: bubble and point. But its stability has a certain limit, so a compensation line is added on the unsaturated iron core, so that the output voltage can reach a high degree of stability.