I. Overview
The purpose of electroplating is to deposit a dense, firm and practical coating on the surface of the electrode. In fact , it is possible to obtain different types of discharge particle deposition through both chemical and electrochemical reactions at the interface. Due to the different shapes and properties of sediments , their applications vary. Several different types of production applications . When it is desired to extract the desired species of atoms from the solution, it is customarily called electrosmelting; if it is required to separate pure substances from materials called electrolytic purification or electrorefining; if the deposited coating Thick and weak bonding with the base material, so it can be separated and directly used as a workpiece is called electroforming; when the deposited material structure is loose and fine, it is currently used to make various powder materials such as powder for powder metallurgy. As for the use of reactions on the interface for oxidation, reduction, catalysis, production of gases, compounds or organic intermediates, promotion of dissolution or surface cleaning, corrosion dissolution or etching, cutting or polishing, surface selective dissolution treatment or formation of conversion coatings Layers, etc., all belong to the production application of this type interface characteristics.
Electroplating, a method of obtaining a strong, dense and structured coating, differs substantially from the above-mentioned applications. However, due to the similarity of ideas, systems and equipment used, although the forms and results are different, they are often mixed in production . This kind of process formed by particle electric exchange on the interface can be summarized as electrochemical production in principle. Because electroplating needs to make the deposited particles form a firm and dense covering, it has its own special rules.
The electroplating process occurs at the electrode interface, so to understand the principle of the deposition process, it is necessary to study the basic reactions at the interface where the ionic conductor and the electronic conductor are in contact and the various reaction steps associated with it. Electronic conductors and ionic conductors have different conduction mechanisms. Therefore, in the electrolysis process, a series of transformation steps are required to realize the discharge of charged particles and transition across phases to be deposited as a coating. First, the deposited particles need to be transported towards the interface in the medium on one side of the interface in order to supply the needs of the deposition process (mass transfer process). Secondly, it can react on the interface, and to complete such a reaction, it is necessary to make some pre-reaction preparations after reaching the interface area to adapt to the exchange on the interface (surface transformation). Electron exchange then takes place at the electrode interface (electrochemical step). Eventually, the discharged particles form a new phase on the surface of the solid phase on the other side of the interface (phase formation).
In the process of generating new phases, the discharged particles can aggregate and nucleate to form growth points, or they can be discharged directly on the active area of the surface. Either the particles are first discharged and captured by the force field of the surface and then move along the surface (surface diffusion) in order to find a suitable position (junction) that may be provided on the substrate surface, or sequential epitaxy along the structure of the surface (epitaxy growth) . In many cases, the formed coating (new phase) and the original substrate may or may not be stabilized depending on the circumstances . The atoms of the new phase may go further into the interior of the substrate (diffusion or inter-diffusion), or undergo a certain reaction (transformation), and may participate in the phase transition of the matrix material, etc.
From the perspective of engineering applications, the coating should at least not be loose or weakly bonded. Otherwise it is worthless. Secondly, it must possess certain useful properties and structure so as to adapt to the required use. Practical metal materials have appropriate structures. Either crystalline or amorphous, the influence of the structure will be manifested in the material properties. The same applies to the plating layer.
2. Plating solution system
Some single metal salts, such as copper sulfate, zinc sulfate, etc., can deposit the corresponding metal deposition layer on the cathode if they are made into an aqueous solution and inserted into the electrode and passed through an electric current . This type of coating is generally difficult to meet the current requirements for the performance, quality or process of the deposition process of the industrial electroplating layer. The simple so-called Daniel (Daniel) battery can be said to be a good example. This early primary battery achieved electrodeposition without an external power source. Due to the difficulty of process control, the coating performance is poor and cannot meet the application requirements.
Therefore, the modern electroplating process uses an ingredient system formulated according to requirements. Generally, the following .
1. Metal supply agent
Soluble salts containing metal ions to be deposited are used to supply metals for deposition. It is customary in the industry to refer to this type of metal salt as the main salt. However, the main salt is to be understood as the salt from which the deposited metal is mainly provided, and not the main salt or the main form present in the solution.
2. Plating solution improver
A single salt is used as a component of the plating solution, and generally the deposited coating cannot meet the usual application requirements, as mentioned above. In order to improve the performance and quality of the deposited coating, and facilitate the monitoring of the process, it is generally necessary to add one or more compounds to adjust the function of the solution. For example, in order to change the form of discharge ions or adjust the potential of discharge, a complexing agent can added; in order to improve the conductivity of the plating solution, a conductive salt can be added; in order to improve the structure of the plating layer, organic additives can be added; in order to stabilize the plating solution, Buffers, stabilizers, etc. may be added.
3. Anode conditioner
The smooth and normal dissolution of the anode is of great importance to the balance and stability of the plating solution in use. The dissolution rate of the anode must be coordinated with the deposition rate of the cathode, otherwise it will affect the material balance of deposited ions. Anodes often have passivation, dust hanging, abnormal loss and other phenomena, which will directly affect the quality of the coating. For example, the anode ash falls off and is suspended in the solution, which will inevitably pollute the bath and be entrained into the coating. Abnormal morphology of anode dissolution can also cause impurity suspension and material waste . If a completely insoluble inert anode is used, the plating solution must be adjusted in time to maintain the normal concentration of ions.
4. Additives for plating solution
Usually in order to adjust one or more indicators, it is necessary to add some substances to the plating solution formula. These substances generally need to be determined through experiments or based on experience, including inorganic substances, organic or natural substances, and artificially synthesized compounds . For example, improving the dispersion ability of the plating solution, refining the coating grains, increasing the smoothness and brightness, and eliminating the pitting of the coating, etc. Since the combination of several substances often has a synergistic and enhanced effect, the additives of products are often used in combination with .
At present, the plating solution used in production is still mainly aqueous solution. Organic solvents or their mixtures with water, molten salts, etc. have also been gradually used in production, especially for the deposition of metals that cannot or are difficult to deposit in aqueous solutions. However, due to the relative difficulty of operating control compared with conventional aqueous solutions, it is not very common. For metals that cannot be precipitated from aqueous solutions , the use of non-aqueous solvents or molten salts is currently the only feasible solution. Some advantages of non-aqueous plating solutions are also promoting the transfer of processes that are accustomed to using aqueous solutions to non-water. Therefore, generally speaking, the plating solution system used for electroplating actually includes water and non-aqueous systems, and also includes a mixed system with some water added.
3. Electrocrystallization process
Plating from aqueous solution is the main method of electroplating production process at present. Electrodeposited coatings are mostly crystalline, including columnar or layered crystalline structures, as well as microcrystalline, nanocrystalline, and amorphous structures. The formation of structures depends on the conditions of the deposition process.
In most cases, coatings commonly used today have a crystalline structure. Since the deposition process is a process , this process is regarded as a crystallization process under the influence of an electric field and is called electrocrystallization. The electrocrystallization process is similar to, but also different from, ordinary crystallization from solution due to supersaturation. The crystalline coating is an ordered structure . A single discharge ion is used to form a crystal lattice, and the ion has a certain regular charge in the solution before discharge . The charge is neutralized by the applied current through electron exchange at the electrode interface. Therefore, the amount of electricity required for neutralization depends on the number of particles and the amount of charge carried by the ion discharge, forming a certain quantitative relationship with each other. This law based on particle counting is expressed by Faraday's law.
Whether the electroplating power supply is constant DC or with ripples, the electricity flowing in the plating tank is expressed as the sum of the product of current and time. During the period from t1 to t2 , the charge

If the current waveform is constant, it can be directly written as

When the flowing current also triggers other side reactions at the same time, the ratio of the electricity Q M actually used to deposit the coating to the total electricity flowing is called the current efficiency.

If the current is completely used to deposit the coating, && !; if the current is completely consumed by side reactions, such as hydrogen evolution, then f=0.
In the actual electroplating process

Current efficiency & is generally expressed as a percentage.
According to Faraday's first law, the mass of the precipitate

And according to the second law

So there is

or

In the formula, M is the molecular weight, and n is the number of charges (valence) transferred by the reaction. The proportional constant F is called Faraday's constant , and its value is equal to the number of particles per mole, that is, the number of Avogadro (Avogadro) multiplied by the electronic charge, which is approximately 96500/mol.
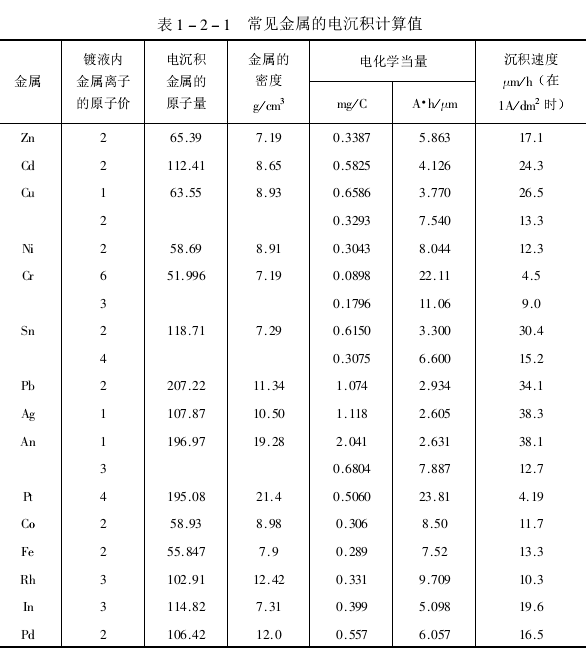
According to formula (2-8), considering the current density during electroplating, the surface area to be plated and the density of the coating, the speed of electroplating or the time required to deposit a certain thickness of coating can be calculated. For a specific plating solution, the current efficiency should also be taken into account. Faraday's constant is a universal constant. However, the seemingly uniform and flat surface in actual engineering calculations is not actually smooth and the current density is not the same everywhere. Therefore, there is often a discrepancy between the theoretically calculated value and the measured value. Easier reference values are estimated by apparent value and average current.
The values of various commonly encountered metals are listed in Table 1-2-1 for reference