The adjusted pH value refers to the acid-base ratio. In chemistry, the negative logarithm of [H + ] is used to express the acidity of the solution, which is called the pH value of the solution. in room temperature:
pH<7, the solution is acidic.
pH=7, the solution is neutral.
pH>7, the solution is significantly subtractive.
Now the actual commonly used indicator is to show the pH value of the solution by the color change of the pH test paper. When the broad-spectrum pH test paper of 0-14 is used, the general relationship between the pH value and the acidity and alkalinity is as follows:
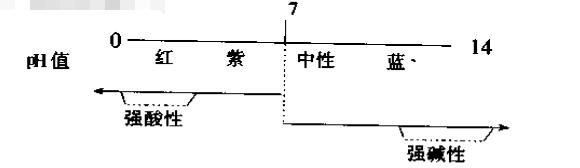
During the test, the color of the pH test paper immersed in the liquid gradually changes from purple to red, which means that the acidity is enhanced; if it changes to blue, the alkalinity is enhanced.
In the coating construction mainly electrophoretic paint coating, when pretreatment with lye degreasing, the degreasing ability of lye increases with the increase of pH value, but when the pH value is greater than 10, it may make aluminum and aluminum alloy products By rotten candle.
At the same time, the pH value is also one of the very important indicators to control the quality of electrophoretic paint. Every kind of electrophoretic paint has a pH value with good water solubility, so it is necessary to strictly control the pH value. At present, the simple method to control the pH value of the electrophoretic paint phase is To control the pH value of the distilled water in the cathode cover (referring to anode electrophoresis), it should be measured frequently. When the pH value increases, the distilled water in the cathode cover must be replaced in time.
From the aspect of construction, the role and significance of pH value are mainly these.