A satisfactory elastomer can quantitatively store and release mechanical energy without loss, that is, it can immediately return to its original shape after deformation. A sympathetic elastic body is measured by its modulus, which expresses its resistance to deformation. The satisfactory viscous body will consume all the mechanical energy received in the form of heat energy. A substance with both elastic and viscous properties is called viscoelastic. Polymer is a typical viscoelastic body, and the paint film composed of polymer is also viscoelastic.
Polymer molecules are subjected to external forces in an instant or in a short period of time, and there is not enough time for relative movement, so they can only change the bond length and bond angle to elongate or deform. Over time, the chain segments of some polymer molecules can move relative to each other and change their relative positions. When the external force acts for a short time, the original shape can be restored after the external force is removed, and the stored energy can be released at the same time, just like an elastic body. If the external force acts for a long time, the external force will be gradually dissipated in the relative movement of the polymer chain segments, and the original shape can no longer be restored. The greater the molecular weight of the polymer, the longer the chains, the greater the degree of intertwining, and the greater the hindrance to the relative movement of the chains.
After the viscoelastic body is deformed by an external force, part of the mechanical energy is stored in the body as potential energy, and part is dissipated as thermal energy (for example, for the relative movement of polymer chain segments). The stress-strain curve of viscoelastic body is related to temperature and time, such as creep and stress relaxation. Creep refers to the gradual increase in deformation with time under constant stress. Stress relaxation refers to the gradual decrease of stress with time under constant strain. The creep or stress relaxation time of viscoelastic body is shortened at high temperature, and prolonged at low temperature, and the effect of time and temperature is the same.
Due to the creep and stress relaxation characteristics of the viscoelastic body, if the stress is a sinusoidal alternating deformation, the strain also changes, but the stress lags behind the strain by a phase angle (δ). The stress lagging behind the deformation is divided into two components, one is in phase with the deformation, that is, γ conδ, and the other is 90° with the deformation, that is, γ sinδ, and thus can be divided into the same phase modulus or the real modulus (E' ), and the modulus or imaginary modulus (E″) with a lag of 90°.
The real modulus is the "elastic" part of the viscoelastic body and is also called the storage modulus because it can be quantitatively stored in the body. The imaginary modulus is the "viscous" part of the viscoelastic body, also known as the dissipation modulus. Since the moduli are dissipated entirely as heat, the relationship of the phase angles to them is:
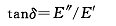
Tanδ is the ratio of "viscosity" to "elasticity" of a viscoelastic body, often called dissipation tangent, internal friction or damping, and is a value that can be measured by dynamic mechanical analysis.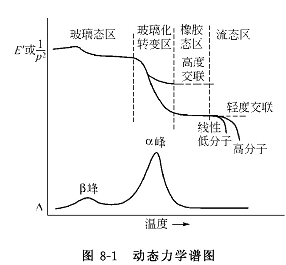
The dynamic mechanical diagram (as shown in Figure 8-1) can be measured with a dynamic mechanical analyzer. There is a peak (α peak) on the high temperature side. The temperature of this peak is equivalent to T g , which is a sudden change in the physical properties of viscoelastic bodies and many other properties . point. There is a peak (peak 13) on the low temperature side of the α peak, which is in the glassy region and is the response of the local motion of the polymer chain segment. The β peak shows the stress relaxation in the glass state, and the paint film with the β peak has better flexibility at low temperature (in the glass state) than the one without the β peak, and is more suitable for lower temperatures. The paint film with excellent flexibility and impact resistance can see the appearance of β peak.
The α peak in the dynamic mechanical spectrum has a strong resolution to the microstructure of the paint film, and is used to guide the synthesis of film-forming polymers and the formulation of paints. Thermoplastic coatings containing two immiscible polymers exhibit two alpha peaks, each corresponding to their respective Tg. When two polymers are miscible with each other, they show a broad alpha peak that is broader than either of the two individual peaks. Random copolymers have a broad alpha peak. Block or graft copolymers will show two α peaks, indicating that the same chain segments in the paint film are aggregated into fine phase domains, dispersed in the continuous phase. The emulsion polymer prepared by two immiscible polymers by gradient dropping process only shows one α peak, while the stepwise dropping process shows two α peaks.
Noise-absorbing and vibration-damping coatings should use the α-peak segment with the largest ratio of dissipation modulus to storage modulus, that is, to widen the α-peak so that it can cover the entire operating temperature range. Several mutually compatible polymers with different Tg can be blended, or rubber-like substances with appropriate cross-link density and capable of covering the entire service temperature range can be used as the paint film.
The paint film prepared by baking a certain car topcoat at different temperatures for 15 minutes was subjected to dynamic mechanical spectrum. When baked at 130°C, the Tg is very low, the storage modulus is also very small, and the mechanical strength such as hardness is very low, which is the performance of the low crosslinking density. When baked at 140℃, the T g increases and the storage modulus also increases. Baked at 150°C, T g and storage modulus are basically the same as those baked at 140°C. After baking at 140°C, the T g and storage modulus drop greatly, which is the performance of degradation due to over-baking. So a good baking temperature is 140°C.
