Raman spectroscopy (Raman)
Elastic scattering and inelastic scattering occur when light is irradiated on the material. The scattered light of elastic scattering has the same component as the wavelength of the excitation light, and the scattered light of inelastic scattering has components longer and shorter than the wavelength of the excitation light, collectively referred to as the Raman effect . The Raman effect is the result of the interaction of photons with optical branch phonons.
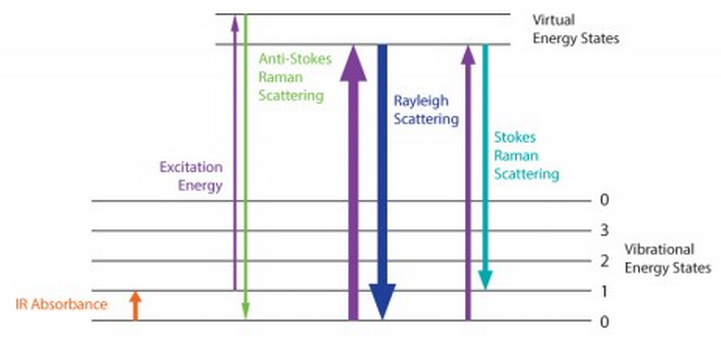
Raman spectrum-principle The Raman effect originates from molecular vibration (and lattice vibration) and rotation, so the knowledge of molecular vibration energy level (lattice vibration energy level) and rotational energy level structure can be obtained from Raman spectrum. The Raman effect can be explained by using the concept of virtual upper level:
Assume that the molecules of the scattering matter are originally in the ground electronic state, and the vibrational energy level is shown in the figure. When irradiated by incident light, the polarization caused by the interaction between the excitation light and the molecule can be regarded as virtual absorption, which is expressed as the transition of electrons to the virtual state (Virtual state), and the electrons on the virtual energy level immediately transition to the lower energy level and To emit light is to scatter light. Assuming that it still returns to the initial electronic state, there are three situations as shown in the figure. Therefore, in the scattered light, there are both spectral lines with the same frequency as the incident light and spectral lines with a different frequency from the incident light. The former are called Rayleigh lines, and the latter are called Raman lines. In the Raman line, the spectral line whose frequency is lower than the incident light frequency is called Stokes line, and the spectral line whose frequency is greater than the incident light frequency is called anti-Stokes line.
The additional frequency value related to the vibration energy level is called the large Raman shift, and the value related to the rotational energy level within the same vibration energy level is called the small Raman shift:
Large Raman shift: (for vibration energy level band frequency)
Small Raman shift: (where B is the rotation constant)
Simple derivation of small Raman shifts: using rotational constants