Ceramic ink is a kind of multi-color ink made of ceramic pigment powder. Small ink droplets are ejected from nozzles with a diameter of tens of microns, and deposited on the body, glaze or other carriers at a speed of thousands of drops per second. Technology.
Ceramic ink is composed of powder (pigment, colorant), solvent, dispersant, binder, surfactant and other auxiliary materials. Ceramic powder is the core and is an inorganic component. The particle size is required to be less than 1 micron. The ink requires a certain solid content, generally not less than 30%, the viscosity is below 100cp, the particle size distribution must be narrow, and there must be no strong agglomeration between particles. It has good stability and is less affected by other substances such as solvents. The dispersant is to ensure that the powder does not agglomerate before printing.
Ink is a very fast process, which requires the ink to be well positioned in its position in a very short time. Before the surfactant has completely migrated to the new interface, the surface tension value must be reduced to a certain value to make the ink wet. Wet body, which requires knowledge of the dynamics of the surfactant before the ink reaches static surface tension. In this way, the printed effect is clear. In addition, the surfactant in the ceramic ink also has the effect of dispersing particles, preventing agglomeration and precipitation. Therefore, the dynamic surface tension is not as low as possible, and a balance needs to be struck between various performance requirements.
1. The concentration of surfactant in the liquid has three situations,
1. The surfactant concentration in the liquid is below the critical micelle concentration (CMC). At this time, the static surface tension decreases with the increase of the surfactant concentration, as shown in the oblique line in the figure below.
2. The surfactant concentration in the liquid is equal to the critical micelle concentration (CMC), at this time, the static surface tension drops to the inflection point. The inflection point in the figure below.
3. The surfactant concentration in the liquid is greater than the critical micelle concentration (CMC). At this time, the static surface tension only drops very slightly, as shown in the horizontal line in the figure below.
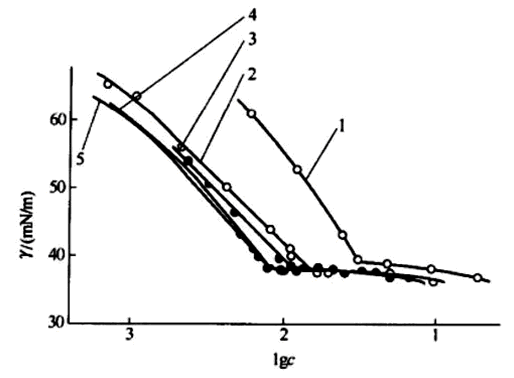
Figure Surface Tension vs. Concentration
The concentration of surfactant in ceramic ink is always greater than the CMC value, so that it can play the role of wrapping particles and wetting. When the static surface and interfacial tensiometer tests the concentration of surface activity greater than CMC, the value changes very little. That is to say, the static Surface Tensiometer cannot reflect the change of surfactant concentration.
1. Static surface tension method
Static surface tension, such as the ring-pull method, is to use a ring initially immersed in the liquid to pull out a liquid film from the liquid, and measure the force that needs to be applied when the ring leaves the liquid surface to calculate the surface tension.
And when the surfactant concentration is greater than the CMC value, the surfactant will not increase the arrangement on the gas-liquid interface, but will form micelles or free states inside the liquid, so the ring-pull method cannot measure when the concentration increases difference in surface tension.

2. Dynamic Surface Tension Method
The German SITA dynamic Surface Tensiometer blows a bubble from the capillary to generate a new gas-liquid interface inside the solution. It takes time for the surfactant in the solution to migrate to the new interface. Above the CMC value, the higher the concentration, the more the surfactant migrates to the new interface, and the lower the measured surface tension value is. The lower the concentration, the less the number of surfactants migrated to the new interface, and the higher the measured surface tension value.
Therefore, using the bubbling dynamic Surface Tensiometer, the surface tension change at different surfactant concentrations can be measured, so as to guide incoming material inspection, research and development, production quality inspection, etc.
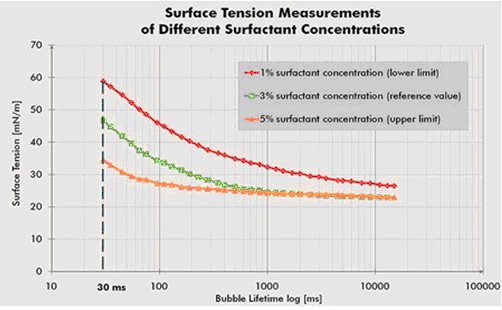
The surface tension change curve under different surfactant concentrations in Fig.






