Fluorescence is a form of luminescence. Luminescence refers to the emission of light after atoms or molecules are stimulated. Objects rely on external light sources for irradiation, and then obtain energy, which produces a phenomenon of excitation and light emission, which is called photoluminescence. such as fluorescence and phosphorescence. But there is a difference between the two. After photons are stimulated, phosphorescence emits outgoing light with equal or lower energy (equal or longer wavelength) than the incident light, and can last up to milliseconds ( ), because the energy supply needs to go through several intermediate steps. Phosphorescence lasts longer than fluorescence in nanoseconds ( ) after the incident light ceases.
The mechanism of fluorescence is shown in Figure 1. To excite the fluorescence, the test surface is illuminated with a UV light source, the high-energy radiation is absorbed by the contaminant molecules on the test surface, and the electrons excited by the photons jump to a higher energy level ( , excited state). The excited molecules collide with their surroundings, releasing a small portion of the absorbed energy.

The remaining energy will return to the original state and release the absorbed energy again, emitting fluorescence. Since part of the energy is converted into heat and consumed, the energy of the emitted light decreases and the wavelength becomes longer. This phenomenon that the emitted fluorescence wavelength is longer than the excitation light and the energy is smaller than the excitation is called displacement.
Fluorescence is emitted in different directions, and through photofading, the characteristics of fluorescence will gradually disappear.
Fluorophore
A fluorophore is a functional group within a molecule that can absorb energy at a specific wavelength and re-emit energy at another wavelength due to the existence of many -systems.
In the - system, two atoms are connected to a molecule through a double compound, that is, each of the two atoms provides two pairs of electrons for combination, and these electrons are easy to excite fluorescence. The wavelength and amount of fluorescent radiation emitted depends on the fluorophore and its chemical environment. Fluorescein is an important fluorescent dye, as shown in Figure 2. Fluorescein contains many double compounds, thus providing the possibility for the molecule to fluoresce remarkably!
Fluorophore fluorescence is characterized by its quantum yield. Quantum yield is defined as the ratio of molecules undergoing photochemical reactions to the total number of photons absorbed, and quantum yield specifies which fraction of the absorbed energy is of such intensity that photons are emitted.
Fluorescence of pollutants
In production tools, the emitted fluorescence is mainly due to aromatic ring systems, ie unsaturated structures containing additives as well as oils and greases. Carboxylic acids and their corresponding esters, as well as aliphatic ketones, also reflect fluorescence. Fluorescence measurement can detect very small amounts of fluorescent substances, even fingerprints with less than 2% sebum can be detected.
The sole exception is substances that cannot fluoresce even when the tube is stimulated by ultraviolet light. These include silicone oils, saturated organic compounds and hydrides of a small amount of unsaturated carbon, metals and their oxides. If the alloy is fluorescently labeled with fluorescent pigments or dyes, non-fluorescent substances can also be detected.
Figure 3 shows the fluorescence spectra of three different oil stains.
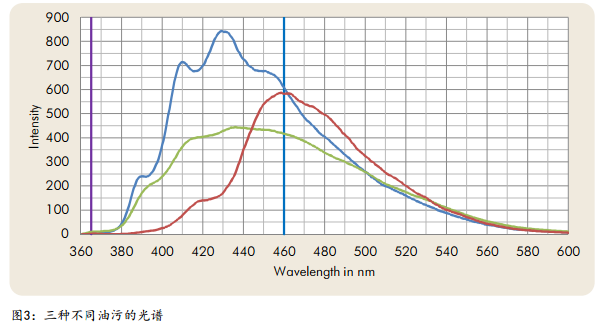
The SITA CleanoSpector surface cleanliness instrument stimulates and detects wavelengths at and wavelengths (marked purple and blue in Figure 3) and is optimized to inspect clean parts of production tools used in industrial production.
Fluorescence of the substrate
Metal and ceramic surfaces do not fluoresce. Contaminants with an amorphous structure may fluoresce on the glass surface. Substrates of other materials, such as paper, textiles or plastics, are quite fluorescent due to their complex organic molecular structures.
photofading
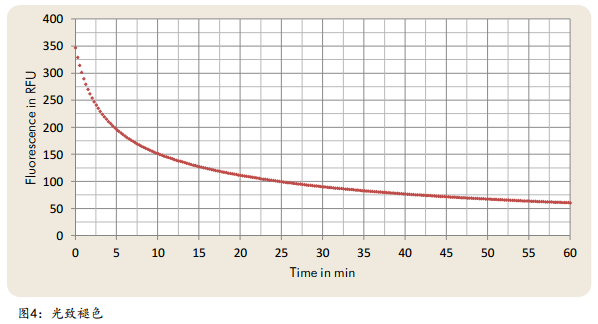
Photofading, also known as photobleaching, refers to the phenomenon that the fluorescence intensity excited by fluorescent substances gradually weakens or even disappears over time under the irradiation of light. Photobleaching describes the permanent loss of fluorescence of a fluorophore due to photon stimulation. During the dynamic process, fluorescent molecules are photochemically destroyed. The reduction in the number of stimulation and emission cycles of a fluorophore due to photobleaching depends on the strength of the stimulus and energy, the molecular structure and the chemical environment. On the basis of oil stains as an example, the dynamic loss of fluorescence after photofading is shown in Figure 4. At the same measurement point, the surface cleanliness meter was used to repeat the measurement on the same measurement point, and the results showed that the fluorescence gradually weakened.
There is a difference between photodenaturation and photobleaching. In photodenaturation, the loss of absorption and fluorescence spectra occurs only at specific wavelengths and in the modified molecular structure of the dye. Photofading strongly alters molecules and the molecules are broken into smaller fragments. In addition, the addition of reducing substances can lead to photoreduction.
When oxygen is incorporated therein, it means that the oxygen is attached to the bis-compounds of external substituents, as well as to the bis-compounds of rings. There is a generation of polar groups and oxidative decomposition of the ring system, which can result in the persistence of this condition if the excited fluorophore interacts in the triplet state for long periods of time.
