What kind of samples are suitable for salt spray test?
How long is 1h salt spray equal to natural corrosion?
There are many evaluation methods for salt spray results, how to choose?
What are the influencing factors in the salt spray test process?
Salt spray corrosion background and mechanism
Salt spray corrosion is a common and very destructive atmospheric corrosion. The atmosphere contains oxygen, humidity, temperature changes and pollutants, which all constitute related corrosion components and factors. The corrosion of metal materials by salt spray is mainly due to the infiltration of conductive salt solution into the metal to undergo an electrochemical reaction, forming a "low potential metal-electrolyte solution-high potential impurity" micro-battery system, and electron transfer occurs.
Anode: The metal loses electrons to become metal cations and enters the solution in the form of hydrated ions while leaving considerable electrons in the metal.
Me+nH2O Me2+ nH2O+2e-
Cathode: The remaining electrons left in the cathode metal, depolarized by oxygen, reduce and absorb electrons, becoming hydroxide ions.
O2+nH2O+4e- 4OH-
Electrolyte: Sodium chloride dissociates to generate sodium ions and chloride ions, and some chloride ions, metal ions and hydroxide ions react to form metal corrosion products.
2Me2++2Cl-+2OH- MeCl2 Me(OH)2
Chloride ions play a major role in the damage process of salt spray corrosion. It has a strong penetrating ability, easily penetrates the metal oxide layer and enters the metal interior, destroying the passive state of the metal. At the same time, chloride ions have very little hydration energy, so they are easily adsorbed on the metal surface, replacing the oxygen in the oxide layer that protects the metal, and destroying the metal.
In addition to chloride ions, the salt spray corrosion mechanism is also affected by oxygen dissolved in the salt solution (essentially a film of salt solution dissolved on the surface of the specimen). Oxygen can cause the depolarization process of the metal surface and accelerate the dissolution of the anode metal. Due to the continuous spraying during the salt spray test, the salt solution film that continuously settles on the surface of the sample keeps the oxygen content close to saturation. The formation of corrosion products expands the volume of the salt solution that penetrates into the metal defect, thus increasing the internal stress of the metal, causing stress corrosion, and causing the protective layer to bulge.
The hazards of salt spray corrosion
Salt spray corrosion will destroy the metal protective layer, make it lose its decoration and reduce its mechanical strength;
Some electronic components and electrical circuits are interrupted due to corrosion, especially in environments with vibration;
When the salt spray falls on the surface of the insulator, the surface resistance will be reduced; after the insulator absorbs the salt solution, its volume resistance will be reduced by four orders of magnitude;
The active parts of mechanical parts or moving parts are due to the generation of corrosion, which increases the friction force and causes the moving parts to be stuck;
Salt spray test classification
The salt spray test is divided into two categories, one is the natural environment exposure test, and the other is the artificially accelerated simulated salt spray environmental test. Compared with the natural environment, the concentration of chloride in the artificial salt spray environment can be several times or dozens of times the salt spray content in the general natural environment, and the corrosion rate is greatly improved. The time for the salt spray test of the product to get the result is also shorter. greatly shortened. For example, if a product sample is tested in a natural exposure environment, it may take 1 year for it to corrode, while it only takes 24 hours to obtain similar results in an artificially simulated salt spray environment.
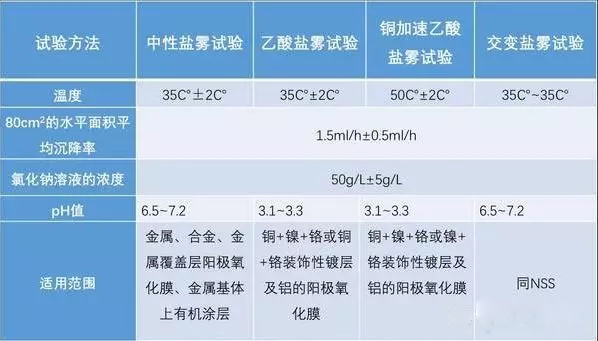
The artificial simulated salt spray test includes neutral salt spray test, acetic salt spray test, copper salt accelerated acetic salt spray test, and alternating salt spray test.
1. Neutral salt spray test
It is an accelerated corrosion test method that appeared earlier and is currently the most widely used. Generally, it uses 5% sodium chloride saline solution, and the pH value of the solution is adjusted in the neutral range (6.5-7.2) as a solution for spraying. The test temperature is set at 35°C, and the sedimentation rate of the salt spray is required to be between 1 and 3ml/80cm2.h, and the sedimentation amount is generally between 1 and 2ml/80cm2.h.
2. Acetic salt spray test
It is developed on the basis of neutral salt spray test. It is to add some glacial acetic acid to the 5% sodium chloride solution, so that the pH value of the solution is reduced to about 3, the solution becomes acidic, and the final salt spray is also changed from neutral salt spray to acidic. Its corrosion rate is about 3 times faster than that of the NSS test.
3. Copper salt accelerated acetic acid salt spray test
It is a rapid salt spray corrosion test recently developed abroad. The test temperature is 50°C, and a small amount of copper salt—copper chloride is added to the salt solution to strongly induce corrosion. Its corrosion rate is about 8 times that of the NSS test.
4. Alternating salt spray test
It is a comprehensive salt spray test, which is actually a neutral salt spray test plus a constant damp heat test. It is mainly used for cavity-type complete machine products. Through the penetration of the humid environment, salt spray corrosion not only occurs on the surface of the product, but also inside the product. It is to alternately switch the product under two environmental conditions of salt spray and humid heat, and finally assess whether the electrical and mechanical properties of the whole product have changed.
Comparison of 4 Salt Spray Test Methods in GB/T 10125
Factors Affecting Salt Spray Testing
The main factors affecting the results of the salt spray test include: test temperature and humidity, the concentration of the salt solution, the angle of sample placement, the pH value of the salt solution, the amount of salt spray settlement and the spraying method, etc.
1. Test temperature and humidity
温度和相对湿度影响盐雾的腐蚀作用。金属腐蚀的临界相对湿度大约为70%。当相对湿度达到或超过这个临界湿度时,盐将潮解而形成导电性能良好的电解液。当相对湿度降低,盐溶液浓度将增加直至析出结晶盐,腐蚀速度相应降低。
试验温度越高盐雾腐蚀速度越快。国际电工委员会IEC60355:1971《AN APPRAISALOFTHE PROBLEMS OF ACCELERATED TESTINGFORATMOSPHERICCORROSION》标准指出:“温度每升高10℃,腐蚀速度提高2~3倍,电解质的导电率增加10~20%”。这是因为温度升高,分子运动加剧,化学反应速度加快的结果。对于中性盐雾试验,大多数学者认为试验温度选在35℃较为恰当。如果试验温度过高,盐雾腐蚀机理与实际情况差别较大。
2、盐溶液的浓度
盐溶液的浓度对腐蚀速度的影响与材料和覆盖层的种类有关。浓度在5%以下时钢、镍、黄铜的腐蚀速度随浓度的增加而增加;当浓度大于5%时,这些金属的腐蚀速度却随着浓度的增加而下降。上述这种现象可以用盐溶液里的氧含量来解释,盐溶液里的氧含量与盐的浓度有关。在低浓度范围内,氧含量随盐浓度的增加而增加,但是,当盐浓度增加到5%时,氧含量达到相对的饱和,如果盐浓度继续增加,氧含量则相应下降。氧含量下降,氧的去极化能力也下降即腐蚀作用减弱。但对于锌、镉、铜等金属,腐蚀速度却始终随着盐溶液浓度的增加而增加。
3、样品的放置角度
样品的放置角度对盐雾试验的结果有明显影响。盐雾的沉降方向是接近垂直方向的,样品水平放置时,它的投影面积最大,样品表面承受的盐雾量也最多,因此腐蚀最严重。研究结果表明:钢板与水平线成45度角时,每平方米的腐蚀失重量为250g,钢板平面与垂直线平行时,腐蚀失重量为每平方米140g。GB/T2423.17-93标准规定“平板状样品的放置方法,应该使受试面与垂直方向成30度角。”
4、盐溶液的pH值
盐溶液的pH值是影响盐雾试验结果的主要因素之一。pH值越低,溶液中氢离子浓度越高,酸性越强腐蚀性也越强。以Fe/Zn、Fe/Cd、Fe/Cu/Ni/Cr等电镀件的盐雾试验表明,盐溶液的pH值为3.0的醋酸盐雾试验(ASS)的腐蚀性比pH值为6.5~7.2的中性盐雾试验(NSS)严酷1.5~2.0倍。由于受到环境因素的影响,盐溶液的pH值会发生变化。
5、盐雾沉降量和喷雾方式
盐雾颗粒越细,所形成的表面积越大,被吸附的氧量越多,腐蚀性也越强。自然界中90%以上盐雾颗粒的直径为1微米以下,研究成果表明:直径1微米的盐雾颗粒表面所吸附的氧量与颗粒内部溶解的氧量是相对平衡的。盐雾颗粒再小,所吸附的氧量也不再增加。
传统的喷雾方法包括气压喷射法和喷塔法,最明显的缺点是盐雾沉降量均匀性较差,盐雾颗粒直径较大。超声雾化法借用超声雾化原理将盐溶液直接雾化成盐雾并通过扩散进入试验区,解决了盐雾沉降量均匀性差的问题,而且盐雾颗粒直径更小。不同的喷雾方法对盐溶液的pH值也会产生影响。
不使用压缩空气的超声雾化法对盐溶液pH值的影响不大,而利用压缩空气喷雾的气压喷射法和喷塔法,盐溶液的pH值变化明显。
盐雾试验结果的表述
盐雾试验的目的是为了考核产品或金属材料的耐盐雾腐蚀质量,而盐雾试验结果判定正是对产品质量的宣判,它的判定结果是否正确合理,是正确衡量产品或金属抗盐雾腐蚀质量的关键。盐雾试验结果的表述有很多种方法,下面简单介绍几种常用的表述方法。
1、按照腐蚀物质的外观特征进行表述
这种方法是According to盐雾试验后腐蚀物的外观特征来进行表述,常见电镀件盐雾试验后腐蚀特征。
2、按照腐蚀面积的百分比进行表述
这种方法适应于平板状样品。如果试验时间较短或样品外形复杂,腐蚀面积较难测定。
ISO 10289 (GB/T 6461)金属基体上金属和无机覆盖层腐蚀评价
保护评级(RP)与外观评级(RA)
式中:A:腐蚀覆盖面积占总面积的百分数;R:保护等级,分为0~10级。
覆盖层分类及主观评价
3、按重量增减表述
这种方法是According to腐蚀物会造成样品重量发生变化,称量样品在试验前后重量变化,分为失重法和增重法。失重法就是使用能够溶解腐蚀物,同时对样品自身又不起化学反应的化学溶剂,把试验后样品上的腐蚀物溶解掉,让试验后样品的重量比试验前轻的一种表述方法。失重法的表示方法为:试验后单位试样面积失去重量的数值。增重法直接测量试验后单位试样面积增加重量的数值。
盐雾测试相关标准
盐雾试验标准是对盐雾试验条件,如温度、湿度、氯化钠溶液浓度和PH值等做的明确具体规定,另外还对盐雾试验箱性能提出技术要求。同种产品采用哪种盐雾试验标准要According to盐雾试验的特性和金属的腐蚀速度及对盐雾的敏感程度选择。
1)盐雾测试标准总结:
ISO 7253-1996(涂料) BS 3900-F12-1997(涂料)
BS 7479:1991 IEC 60068-2-11:1981
GB/T 10125-1997(涂料) GB 2423.17-2008
DIN 50021-1988
2)酸性盐雾测试标准:
ASTM B368-09 ISO 9227-2006
DIN 50021-1988 BS 7479:1991
3)铜离子加速盐雾测试标准
ASTM B368-09 ISO 9227-2006
DIN 50021-1988 BS 7479:1991
4)循环盐雾测试标准
ASTM D6899-2003 ASTM G85-02e1 Annex A5
ISO 11997-1:2005 ISO 11997-2:2000 SAE J2334:2002
WSK-M2G299 GM4298P GM4476P GM9540P
盐雾设备展示
Salt spray test is an important means to assess the salt spray corrosion resistance of products or materials, and the importance of the scientific and rationality of the test results goes without saying. There are many factors that affect the stability and consistency of salt spray test results. To improve the validity of salt spray test results, test technology is the key. Therefore, test personnel not only need to have solid professional knowledge and professional skills, but also need rich practical experience and multi-faceted understanding of products, to understand salt spray test from multidisciplinary fields such as chemical and environmental engineering, materials, structure and technology, scientific Reasonably express the test results, better provide effective information for product material selection, structural design, process selection, product transportation, storage and use, and improve the salt spray corrosion resistance of products or materials.
