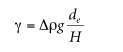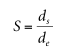Surface tension is defined as the excess force per unit length on a surface; if it is considered positive. Act in the direction of surface contraction. 1,6 The tendency for systems to reduce their surface area is the result of excess surface energy because surface atoms are subjected to different environments. compared to bulk cargo. The surface tension of liquids and polymer melts can be measured by methods such as capillary, 1 Dunoir ring, 2,7 Wilhelmy plate, 8 and hanging drop. We should focus on 5. We discussed two methods: the capillary method and the pendant drop method.
The capillary height method is a suitable method for low viscosity liquids because the system takes a long time for high viscosity liquids to reach equilibrium. According to reports, it takes 4 days.
To bring the polystyrene melt to equilibrium at 200 °C, 5 Figure 2.1 illustrates the capillary height method.
In equilibrium, the forces acting on the perimeter of the meniscus due to surface tension need to be balanced.
By the weight of the liquid column. Neglecting the weight of the fluid above the meniscus, approximately— the solution equation can be written as follows:
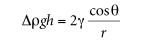
where Δρ is the density difference between the liquid and air, G is the gravitational constant, h is the height of the liquid column, γ is the surface tension, θ is the contact angle, and R is the radius of the capillary. In practice, it is difficult to measure the vertical contact angle precisely and is known and uniform.
radius. In order to more accurately measure surface tension, various methods can be used.
Calculate the weight of the liquid above the liquid surface.
The pendant drop method is a very versatile technique for measuring the surface tension of liquids.
Interfacial tension between two liquids. Andreas et al. 9 Measure the surface in this way.
Tension of various organic liquids. Wu 10 and Roe 11 have widely applied this method to measure
Surface and interfacial tension of many polymeric liquids and melts.
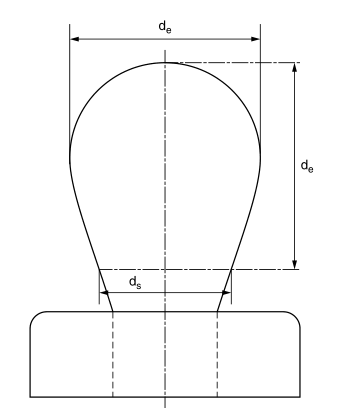
In a selected plant, the diameter of the droplet is at the distance D from the apex.
Pull down (see Figure 2.3). A table of 1/h values as a function of s can be displayed. 12–14
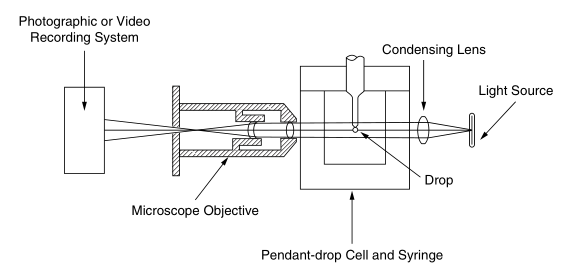
Recently, there have been some major improvements in data acquisition and analysis. About weeping drop sections. 15 - 17 of the photographic recordings and measurements of the drops are directly digitally replaced by video images. The ability to measure the entire drop profile led to the development of a new algorithm for drop profile analysis