thickness
Electrolysis is applied on top of a very thin metal layer. The electrolysis process produces no sharp buildup at corners or edges; it conforms perfectly to the surface. There was no change in the conductivity or magnetism of the base metal.
Electrowinning ranges from 0.000010 to 0.003. each side. Actual coating range is 0.000025 to 0.01 inches. The average deposit rate is £0.0004 to £0.0008. each side. Thickness tolerance is 0.000010 to 0.000050. Maintenance can be performed according to the specified thickness and quality level of the part. Coating thickness may exceed 0.001 inch. can be applied, and tolerances can be maintained after eliminating electrolize grinding operations. Tables 29.2 and 29.3 list thickness ranges and tolerances for most metals. Recommended coating thicknesses and tolerances are established by consulting electrolytics.

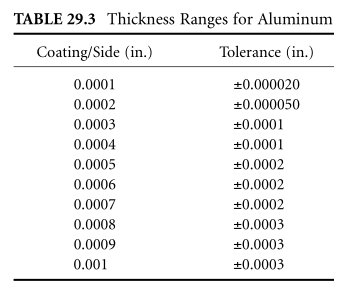
Electrolytic thickness is always in direct relation to the base metal and will vary from one base metal to another. However, the thickness established for each will remain constant and predictable. In all applications requiring monitoring of deposit thickness, Close Inspection Electrolysis, Inc., recommends the use of a rigorous anti-corrosion agent to determine deposit thickness. This method is non-destructive to the base metal. If necessary, micrographs of the damage will verify and conform to the plating thickness.
Adhesion
Electrolytic has excellent adhesion properties. The adhesion of the coating is such that, when examined at four diameters, it will not show separation from the base metal on repeatedly bending the specimen at 180°, with diameters equal to the thickness of the specimen, until fracture.
Through the surface porosity of the base metal, the electrolytic coating forms a durable bond with the surface , but it can be detrimentally eliminated by empowering the electrolysis of the base metal.
corrosion
Electrolysis resists attack by most organic and inorganic compounds (except sulfuric and hydrochloric acids). The electrolytic coating is usually more noble than the substrate; therefore, it prevents corrosion without porosity, cracks, and discontinuities, and provides a uniform structure and chemical composition. Porosity, hardness, and surface finish of the base metal will affect electrolytic corrosion performance; however, it is the electrolize coating that can enhance the corrosion resistance characteristics of all base metals.
Samples meet standard ASTM B-117 and B-287 salt spray tests. The electrolysis process also meets the following specifications: QQ–C–320, AMS–2406 mil–C–23422 mil–P–6871, ANP–39, NASAND-1002176.
Wear resistance (surface hardness)
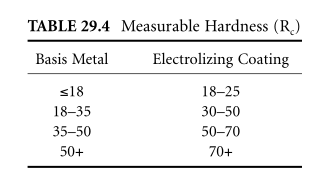
Electrolysis is a difficult chrome surface, measuring 70 to 72 RC, applied. "Application" refers to the application of electrolytic coatings to metals on the basis of measurements when measuring hardness. The base metal plays an important role in determining the wear resistance of electrolytic surfaces. In general, increase the measured electrolytic hardness by 10 to 15 points, as shown in Table 29.4.
In all cases, electrolytic 70 72 RC. However, the base metal directly affects the measurable hardness. Based on the electrolysis of hard metals, high hardness can be obtained. The surface hardness test shall be measured by the Knoop or Vickers method with a load of 5 to 10 grams at a diamond point.
A high hardness value indicates good wear resistance, but there are other factors to consider, such as coating surface texture, coating density, substrate cleaning before coating, bond interface energy between coating and substrate, coating surface and relative sliding The adhesive energy between materials, the type of lubricant used, and the opposing material combination.
Increased density improves wear resistance as it results in fewer cracks, inclusions and voids, which reduces the rate of corrosive attack and provides more resistance to chipping, spalling and wear.
Surface fatigue wear - the category of true spalling - also affects the wear resistance of traditional QQ-C-320 chrome plating. Here the stress concentration is a phenomenon produced during the electrodeposition process, when the bulk chromium crystals are formed. The electrolytic chromium alloy coating is substantially free of these internal stress concentrations and therefore has a minimal tendency to spall. This is the reason for a substantial advantage over conventional chromium plating in terms of wear resistance under electrolytic mechanical impact conditions.
The electrolytic coating also has a low coefficient of friction, wear resistance and is superior to all other chromium coatings (including electroless nickel and tungsten carbide coatings), measured on three different testing machines: Taber, Falex, and LFW–1.
Finally, the electrolytic coating has extremely low adhesive wear coefficients: 1.72×101–7 steel-copper alloy, 1.19×10–7 steel-on-steel and oil (10–8 accounts for final or very good wear coefficients measured).
Lubricity
An acidic coating has a coefficient of friction of 0.11. Electrolytically produced dynamic coefficients of friction as low as 0.045 for one-way sliding test conditions with fluorosilicone oil under LFW–1 test machine, and 0.069 for additive-free white mineral oil in Falex lubricant. Electrolytic's low coefficient of friction is invaluable in relation to extreme temperatures.
consistency
Electrolysis works better when applied to a relatively smooth surface (12 to 32 RMS or finer). Below an RMS of 4, the process may be slightly hindered by the surface finish required after the electrolytic operation.
Inner and outer surfaces of nearly all shapes and configurations can be machined uniformly. Grooves or grooves smaller than 0.187 inches. Wide, deeper than wide, and less than 0.187 inches in diameter. Diameters will require special engineering to ensure an even coating. For electrolytics, it is recommended to test run the other sections to discuss with an engineer for electrolytic special sizes less than 0.187.
Thermal resistance
The maximum operating temperature recommended for electrolize coatings is approximately 1600°F (710°C). The time at which electrolysis should be performed prior to testing or specifying electrolysis. Generally, oxidation occurs around 1100°F (430°C), progresses to 1650°F (740°C), and then diffuses.
brightness
The surface of the electrolytic coating is smooth, continuous, fine-grained, and the thickness and appearance of the wall are uniform, without blisters, pits, nodules, porosity and edge enhancement. Electrolysis as the final coating of parts and equipment. Gloss application of electrolytic coatings. However, a satin (matte) finish can be achieved if specified.
hydrogen embrittlement
During conventional chrome plating, a harmful side effect occurs: hydrogen blocking. Hydrogen penetrates into the matrix, leading to embrittlement of the metal components and subsequent reduction of mechanical properties, especially fatigue strength. Most traditional chrome control documents, therefore, specify a final 375°F bake to remove hydrogen.
The longer the plating cycle, the greater the possibility of hydrogen embrittlement. Embrittlement is more likely to occur after pickling. Shot peening and/or liquid honing can be used to relieve embrittlement stresses.
Hydrogen embrittlement is highly unlikely to be due to electrolysis, as electrolytic treatment avoids most of the true causes of hydrogen embrittlement. Electrolysis does not include post-processing process baking. However, if handling roasting is required by the client, the electrolytic company, it can be made a standard procedure.









