In 1643, the Italian physicist Torricelli demonstrated the atmospheric pressure experiment, revealing the existence of the physical state of "vacuum" for mankind. In the following centuries, especially at the beginning of the 20th century, vacuum technology developed rapidly and was widely used in military and civilian fields. Similarly, vacuum technology is also the basis for thin film preparation. Almost all thin film materials are prepared under vacuum or under low pressure conditions. Therefore, in this chapter, we will give a brief introduction to some basic knowledge of vacuum, the acquisition of vacuum, and the measurement of vacuum.
Section 1 Basic knowledge of vacuum
1. The unit of vacuum degree
The vacuum in contact with human beings can be roughly divided into two types: one is the vacuum existing in the universe, which is called "natural vacuum"; the other is the vacuum obtained by people using a Vacuum Pump to adjust the gas in the container, which is called "natural vacuum". It is called "artificial vacuum". Regardless of the type of vacuum, as long as the gas pressure is lower than one atmospheric pressure in a given space, it is called a vacuum. The state of space without gas at all is often referred to as an absolute vacuum. "Vacuum" in the general sense does not mean "no matter exists". Currently, even at the lowest pressures achievable with specialized vacuum preparation methods, there are still several hundred gas molecules per cubic centimeter of volume. Therefore, the vacuum we usually refer to refers to a relative vacuum state. In vacuum technology, the idiom "vacuum degree" and the physical quantity "pressure" are commonly used to indicate the degree of vacuum in a certain space, but their physical meanings should be strictly distinguished. The lower the pressure in a certain space, the higher the vacuum, and conversely, the lower the vacuum in a space with high pressure.
"Millimeter of mercury (mmHg)" is an earlier and widely used pressure unit by humans. It obtains the size of the vacuum by directly measuring the length. Especially when using a Torricelli barometer, it is more intuitive to use millimeters as a pressure measurement. But in 1958, mmHg was replaced by "Torr" in honor of Torricelli. 1 Torr refers to the pressure of 1 mmHg on a unit area under standard conditions, expressed as 1Torr = 1mmHg. In 1971, the International Conference on Metrology officially determined "Pascal" as the international unit of gas pressure, 1Pa = 1 N/m2 ≈ 7.5×10-3Torr. Table 1-1 shows the pressure units commonly used in vacuum technology and the conversion relationship between them.
Table 1-1 Conversion relationship of several pressure units

Second, the division of the vacuum area
In order to study the vacuum and facilitate the actual use, the vacuum is often divided into the following areas according to the different physical characteristics in each pressure range:
Rough vacuum: 1×105 ~ 1×102 Pa
Low vacuum: 1×102 ~ 1×10-1Pa
High vacuum: 1×10-1 ~1×10-6Pa
Ultra-high vacuum: <1×10-6Pa
The motion properties of gas molecules in different regions of vacuum are different. In a rough vacuum, the gaseous space is approximately atmospheric, and the molecules are still dominated by thermal motion, and the collisions between molecules are very frequent; in a low vacuum, the flow of gas molecules gradually transitions from a viscous flow state to a molecular state. It is similar to the number of collisions between molecules and the wall; when high vacuum is reached, the flow of gas molecules has become a molecular flow, and the collisions between gas molecules and the container wall are the main ones, and the number of collisions is greatly reduced. The particles of evaporated materials will fly in a straight line; in ultra-high vacuum, the number of gas molecules is less, there is almost no collision between molecules, and there are fewer chances of collision between molecules and the wall.
3. Adsorption of gas by solid and desorption of gas
In vacuum technology, various gases are often encountered, and the adsorption and desorption of these gases on the solid surface is very common. This is an important issue for high vacuum technology, especially ultra-high vacuum technology. question of meaning. For example, in order to increase the vacuum degree in the tube, the parts need to be degassed in advance. This process is the process of desorption of gas molecules on the solid surface. With the desorption of gas, a certain degree of vacuum will be formed in the container. In addition, in vacuum equipment, the principle of adsorption is often used to make various adsorption pumps to obtain high vacuum, and sometimes the ability of clean surfaces to adsorb a large number of gas molecules is used to obtain vacuum.
The so-called gas adsorption is the phenomenon that gas molecules are captured on the solid surface. Adsorption is divided into physical adsorption and chemical adsorption. Among them, physical adsorption is not selective, and any gas can occur on the solid surface, mainly caused by the mutual attraction between molecules. Physically adsorbed gas is prone to desorption, and this adsorption is only effective at low temperatures; chemical adsorption occurs at higher temperatures, similar to chemical reactions, gas is not easy to desorb, but only when the gas and solid surface atoms contact to form Adsorption occurs only when the compound is present. The desorption of gas is the reverse process of gas adsorption. Usually, the process of releasing gas molecules adsorbed on the solid surface from the solid surface is called gas desorption.
In vacuum technology, the phenomenon of adsorption and desorption of gas on the solid surface always exists, but the degree of adsorption or desorption is different under different external conditions. Generally, the main factors affecting the adsorption and desorption of gas on the solid surface are the pressure of the gas, the temperature of the solid, the density of the gas adsorbed on the solid surface, and the properties of the solid itself, such as the degree of surface finish and cleanliness. When the temperature of the solid surface is high, gas molecules are prone to desorption. Proper baking of the vacuum chamber is beneficial to the acquisition of vacuum. This is the reason. In addition to the above effects, in some Vacuum Pumps and vacuum gauges with ionization phenomenon, there are different degrees of electric absorption and chemical scavenging, and these two factors will also accelerate the adsorption of solids to gases. Among them, electroabsorption refers to the formation of positive ions after ionization of gas molecules. Positive ions have stronger chemical activity than neutral gas molecules, so they often form physical or chemical adsorption with solid molecules; , titanium, etc.) occurs when the vacuum evaporation of solid materials occurs, and these evaporated solid materials will form compounds with non-inert gas molecules, resulting in chemical adsorption.
Section 2 Acquisition of Vacuum
The acquisition of vacuum is what people often call "Vacuum Pumping", that is, to use various Vacuum Pumps to pump out the gas in the pumped container, so that the pressure of the space is lower than one atmospheric pressure. At present, the equipment commonly used to obtain vacuum mainly includes rotary mechanical Vacuum Pumps, oil diffusion pumps, composite molecular pumps, molecular sieve adsorption pumps, titanium sublimation pumps, sputter ion pumps and cryogenic pumps. Among them, the first three Vacuum Pumps belong to gas transmission pumps, that is, the purpose of exhaust is achieved by continuously inhaling and discharging gas from the Vacuum Pump; the latter four Vacuum Pumps belong to gas capture pumps, which are a kind of suction pump that uses the unique suction effect of various suction materials. The gas in the evacuated space is sucked to achieve the required vacuum degree. Because these capture pumps do not use oil as a medium when they work, they are also called oil-free pumps. Table 1-2 lists the working pressure range of several commonly used Vacuum Pumps and the ultimate pressure that can usually be obtained. The ultimate pressure is one of the important parameters to express the performance of the Vacuum Pump. It refers to the minimum pressure when the standard container is used as the load and the pump works normally under the specified conditions for a period of time, and the vacuum degree does not change and tends to be stable. The dotted line in the table indicates the area that can be expanded when the Vacuum Pump is used in combination with other devices.
Table 1-2 Working pressure range of several commonly used Vacuum Pumps

从表中可以看出,代表真空度的压强其变化范围在十几个数量级,如果从大气开始抽气,仅使用一种真空泵是很难达到超高真空度的,即没有一种真空泵可以涵盖从大气压到10-8Pa的工作范围。人们常常把2~3种真空泵组合起来构成复合排气系统以获得所需要的高真空。例如,有油真空系统中,油封机械泵(两极)+油扩散泵组合装置可以获得10-6~10-8Pa的压强;无油系统中,采用吸附泵+溅射离子泵+钛升华泵装置可以获得10-6~10-9Pa的压强;有时也将有油、无油系统混用,如采用机械泵+复合分子泵装置可以获得超高真空。其中机械泵和吸附泵都是从一个大气压力下开始抽气,因此常将这类泵称为“前级泵”,而将那些只能从较低的气压抽到更低的压力下的真空泵称为“次级泵”。本节将重点介绍机械泵、复合分子泵和低温泵的结构和工作原理等方面的内容。
一、旋片式机械真空泵
凡是利用机械运动(转动或滑动)以获得真空的泵,就称为机械泵。它是一种可以从大气压开始工作的典型的真空泵,既可以单独使用,又可作为高真空泵或超高真空泵的前级泵。由于这种泵是用油来进行密封的,所以又属于有油类型的真空泵。这类机械泵常见的有旋片式、定片式和滑阀式(又称柱塞式)几种,其中以旋片式机械泵最为常见。
旋片式真空泵是用油来保持各运动部件之间的密封,并靠机械的办法,使该密封空间的容积周期性地增大,即抽气;缩小,即排气,从而达到连续抽气和排气的目的。图1-1是单级旋片泵的结构图,泵体主要由定子、转子、旋片、进气管和排气管等组成。定子两端被密封形成一个密封的泵腔。泵腔内,偏心地装有转子,实际相当于两个内切圆。沿转子的轴线开一个通槽,槽内装有两块旋片,旋片中间用弹簧相连,弹簧使转子旋转时旋片始终沿定子内壁滑动。
图1-1所示,旋片2把泵腔分成了A、B两部分,当旋片沿图中给出的方向进行旋转时,由于旋片1后的空间压强小于进气口的压强,所以气体通过进气口,吸进气体,如图1-2(a)所示;图1-2(b)表示吸气截止。此时,泵的吸气量达到最大,气体开始压缩;当旋片继续运动到1-2(c)所示的位置时,气体压缩使旋片1后的空间压强增高,当压强高于1个大气压时,气体推开排气阀门排出气体;继续运动,旋片重新回到图1-1所示的位置,排气结束,并重新开始下一个吸气、排气循环。单级旋片泵的极限真空可以达到1Pa,而双级旋片泵的极限真空可以达到10-2Pa数量级。

图1-1 旋片泵结构示意图

图1-2 旋片泵工作原理图
由于泵工作时,定子、转子全部浸在了油中,在每一吸气、排气周期中将会有少量的油进入到容器内部,因此要求机械泵油要具有较低的饱和蒸气压及一定的润滑性、粘度和较高的稳定性。
二、复合分子泵
分子泵是旋片式机械真空泵的一大发展。同机械泵一样,分子泵也属于气体传输泵,但是它是一种无油类泵,可以与前级泵构成组合装置,继而获取超高真空。目前,可把分子泵分为牵引泵(阻压泵)、涡轮分子泵和复合分子泵三大类。其中,牵引泵在结构上更为简单,转速较小,但压缩比大;涡轮式分子泵又可以分成“敞开”叶片型和重叠叶片型。前者转速高,抽速也较大,后者则恰好相反。复合型分子泵将涡轮分子泵抽气能力高的优点和牵引分子泵压缩比大的优点结合在一起,利用高速旋转的转子携带气体分子而获得超高真空。图1-3为其结构示意图,

图1-3 分子泵结构示意图
该泵转速24000转/分,第一部分是一个只有几级敞开叶片的涡轮分子泵,第二部分是一个多槽的牵引分子泵,抽速460L/s,转速为零时的压缩比为150。
三、低温泵
低温泵是利用20K以下的低温表面来凝聚气体分子以实现抽气的一种泵,是目前具有最高极限真空的抽气泵。它主要用于大型真空系统,如高能物理、超导材料的制备、宇航空间模拟站等要求高清洁、无污染、大抽速、高真空和超高真空等场合。低温泵又称冷凝泵、深冷泵。按其工作原理又可分为低温吸附泵、低温冷凝泵、致冷机低温泵。前两种泵直接使用低温液体(液氮、液氦等)来进行冷却的,成本较高,通常仅作为辅助抽气手段;致冷机低温泵是利用制冷机产生的深低温来进行抽气的泵,其基本结构如图1-4所示。在制冷机的第一级冷头上,装有辐射屏和辐射挡板,温度处于50-77K,用以冷凝抽除水蒸汽和二氧化碳等气体,同时还能屏蔽真空室的热辐射,保护第二级冷头和深冷板;深冷板装在第二级冷头上,温度为10-20K,板正面光滑的金属表面可以去除氮、氧等气体,反面的活性碳可以吸附氢、氦、氖等气体。通过两极冷头的作用,可以达到去除各种气体的目的,继而获取超高真空状态。
低温泵作为捕获泵,能用来捕集各种包括有害的或易燃易爆气体,使其凝结在制冷板上,以达到抽气的目的。但是,工作一段时间后,低温泵的低温排气能力会降低,因此需要经“再生”处理,即清除低温凝结层。再生时需要遵循以下几点要求:
(1)一旦开始再生处理,就需要清除有效。这是因为局部升温时会使屏蔽板上冷凝的大量水蒸气转移到内部的深冷吸气板上,严重损害低温泵的抽气能力。
(2)再生时应使凝结层稳定蒸发,一定不能使系统内气体压力超过允许值,否则在除氢这类易燃易爆的气体时,一旦漏入空气就有爆炸的危险。
(3)再生时,需严防来自前级泵的碳氢化合物进入低温泵内污染吸气面,因此要求抽气时间尽可能短。
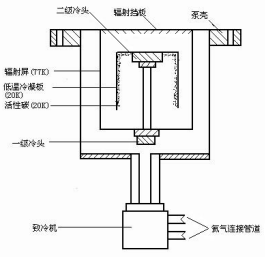
图1-4 低温泵结构示意图
第三节 真空的测量
真空测量是指用特定的仪器和装置,对某一特定空间内真空高低的测定。这种仪器或装置称为真空计(仪器、规管)。真空计的种类很多,通常按测量原理可分为绝对真空计和相对真空计。凡通过测定物理参数直接获得气体压强的真空计均为绝对真空计,例如U型压力计、压缩式真空计等,这类真空计所测量的物理参数与气体成分无关,测量比较准确,但是在气体压强很低的情况下,直接进行测量是非常困难的;而通过测量与压强有关的物理量,并与绝对真空计比较后得到压强值的真空计则称为相对真空计,如放电真空计,热传导真空计,电离真空计等,它的特点是测量的准确度略差,而且和气体的种类有关。在实际生产中,除真空校准外,大都使用相对真空计。本节主要对电阻真空计、热偶真空计、电离真空计的工作原理、测量范围等进行介绍。
一、电阻真空计
电阻真空计是热传导真空计的一种,它是利用测量真空中热丝的温度,从而间接获得真空度的大小的。其原理是低压强下气体的热传导与压强有关,所以如何测量温度参数并建立电阻与压强的关系就是电阻真空计所要解决的问题。
电阻真空计的结构如图1-5所示。规管中的加热灯丝是电阻温度系数较大的钨丝或铂丝,热丝电阻连接惠斯顿电桥,并作为电桥的一个臂。低压强下加热时,灯丝所产生的热量Q可以表示为:
Q = Q1 + Q2
式中Q1是灯丝辐射的热量,与灯丝的温度有关;Q2是气体分子碰撞灯丝而带走的热量,大小与气体的压强有关。当热丝温度恒定时,Q1是恒量,即热丝辐射的热量不变。在某一恒定的加热热丝电流条件下,当真空系统的压强降低,即空间中气体的分子数减少时,Q2将随之降低,此时灯丝所产生的热量将相对增加,则灯丝的温度上升,灯丝的电阻将增大,真空室的压强和灯丝电阻之间的存在这样的关系P↓→ R↑,所以可以利用测量灯丝的电阻值来间接地确定压强。

图1-5 电阻真空计
电阻真空计测量真空的范围是105~10-2Pa。由于是相对真空计,所测压强对气体的种类依赖性较大,其校准曲线都是针对干燥的氮气或空气的,所以如果被测气体成分变化较大,则应对测量结果做一定的修正。另外,电阻真空计长时间使用后,热丝会因氧化而发生零点漂移,因此在使用时要避免长时间接触大气或在高压强下工作,而且往往需要调节电流来校准零点位置。
二、热偶真空计
图1-6为热偶真空计的结构示意图。热偶真空计的规管主要由加热灯丝C与D(铂丝)和用来测量热丝温度的热电偶A与B(铂铑或康铜-镍铬)组成。热电偶热端接热丝,冷端接仪器中的毫伏计,从毫伏计中可以测出热偶电动势。测量时,热偶规管接入被测真空系统,热丝通以恒定的电流,同电阻真空计不相同的是,此时灯丝所产生的热量Q有一部分将在灯丝与热偶丝之间传导散去。当气体的压强降低时,热电偶接点处温度将随热丝温度的升高而增大,同样,热电偶冷端的温差电动势也将增大,及气体压强和热电偶的电动势之间存在这样的关系:P↓→ ε↑。

图1-6 热偶真空计
热偶真空计对不同的气体的测量结果是不同的,这是由于各种气体分子的热传导性能不同,因此在测量不同的气体时,需进行一定的修正。表1-3给出了一些气体或蒸汽的修正系数。
表1-3 常见气体和蒸汽的修正系数

热偶真空计的测量范围大致是102~10-1Pa,测量压强不允许过低,这是由于当压强更低时,气体分子热传导逸去的热量很少,而以热丝、热偶丝的热传导和热辐射所引起的热损失为主,则热电偶电动势的变化将不是由于压强的变化所引起。
热偶真空计具有热惯性,压强变化时,热丝温度的改变常滞后一段时间,所以数据的读取也应随之滞后一些时间;另外,和电阻真空计一样,热偶计的加热灯丝也是钨丝或铂丝,长时间使用,热丝会因氧化而发生零点漂移,所以使用时,应经常调整加热电流,并重新校正加热电流值。
三、电离真空计
电离真空计是目前广泛使用的真空测量计,它是利用气体分子电离的原理进行真空度测量的。According to气体电离源的不同,又分为热阴极电离真空计和冷阴极电离真空计,前者又分为普通型热阴极电离计、超高真空热阴极电离计和低真空热阴极电离计。图1-7给出了普通电离计规管的结构,它主要有三个电极:发射电子的灯丝作为发射极A,螺旋型加速并收集电子的栅极(又称加速极)B和圆筒型离子收集极C等三部分组成,其中发射极接零电位,加速极接正电位(几百伏),收集极接负电位(几十伏),B和C之间存在拒斥场。电离计的工作原理是热阴极A发射电子,经过加速极加速,大部分电子飞向收集极,在B-C之间的拒斥场作用下,电子运动速度降低,当速度减到零时,电子又重新飞向B极,在电子飞向B-C空间时,同样也受到拒斥场的作用,在速度减为零时,电子返转飞向C极,电子在B-C空间的反复运动,将与气体分子不断发生碰撞,使气体分子获得能量而产生电离,电子最终被加速极收集,而电离产生的正离子则被收集极接受并形成离子流I+,对于某一规管,当各电极电位一定时,I+与发射电子流Ie、气体的压强有如下的线性关系
I+ = kIeP
式中k为比例常数,其意义是单位电子电流和单位压强下所得到的离子的电流值,单位为1/Pa,可以通过实验确定。对于不同气体,k的大小不同,其存在的范围在4-40之间。当发射电流一定时,离子流只与气体的压强成正比,因此可以According to离子流的大小来确定真空室中气体压强值。

图1-7 电离真空计
The measurement range of ordinary hot cathode vacuum gauges is 1.33×10-1—1.33×10-5Pa, no matter whether it is higher or lower than this measurement limit, the linear relationship between ion current I+ and gas pressure will be lost. When the pressure is high, the probability of multiple collisions between electrons and molecules is greatly increased. Since the accelerating potential is much higher than the ionization potential (tens of volts) of the gas, the electrons generated by ionization are enough to cause gas ionization. In this way, the ionization regulation The electron flow in the gas increases sharply. At the same time, due to the high gas density, the free path of the electrons is very short. Most of the collisions are low-energy collisions and cannot cause ionization. Many factors cause the linear relationship between the ion flow and the pressure to no longer be maintained at higher pressures. ; When the pressure is low (lower than 1.33×10-1Pa), the high-speed moving electrons will generate soft X-rays when they reach the accelerator, and the soft X-rays will then shoot to the ion collector C, which will cause the collector to generate photoelectric emission. The electron flow is emitted, so that the pressure-independent current is superimposed in the original ion flow measurement circuit, and the linear relationship between the ion flow I+ and the gas pressure is lost. At this time, the ionization vacuum gauge cannot measure the pressure in the vacuum chamber. up.
The ionization vacuum gauge can quickly and continuously measure the total pressure of the gas to be measured, and the gauge is small in size and easy to connect. However, the emitter in the gauge is made of tungsten wire. When the pressure is higher than 10-1Pa, The service life of the gauge will be greatly reduced, or even burnt, and work under high pressure should be avoided; when the vacuum system is exposed to the atmosphere, the inner surface of the glass bulb of the ionization gauge gauge and each electrode will absorb gas, which will affect the accuracy of vacuum measurement , Therefore, when the vacuum system is exposed to the atmosphere for a long time or used for a period of time, regular degassing treatment should be carried out regularly.
references
(1) Zhao Baosheng edited: "Vacuum Technology", Science Press, Beijing, 1998
(2) Yang Bangchao and Wang Wensheng: Thin Film Physics and Technology, University of Electronic Science and Technology Press, Chengdu, 1994
