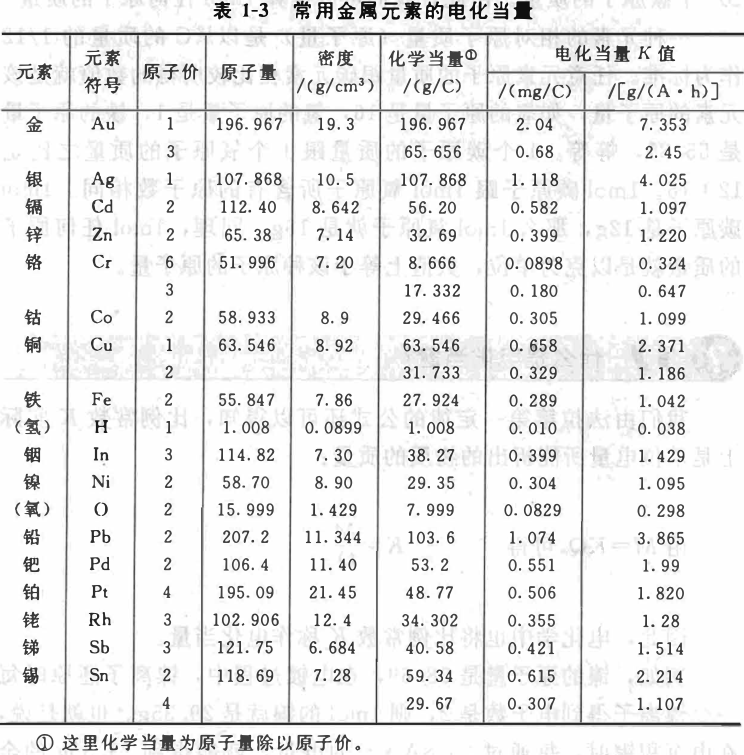
We can also know from the formula of Faraday's first law that the proportionality constant K is actually the mass of the substance that can be precipitated per unit of electricity:
From M=KQ, we can get
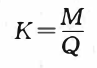
Therefore, the constant of proportionality K is also called the electrochemical equivalent in electrochemistry. For example, the atomic weight of baht is 58.69. During the electroplating process, when the specular ion is reduced, the number of electrons obtained by each bromide ion is 2, so 1mol bromide is 29.35g. That is to say, when electrolytically depositing baht, 29.35g of metal . From this we can deduce that the electrochemical equivalent of baht is:
9. 35g/96500C= 0. 000304g/C
It should be reminded that the value of electrochemical equivalent varies . For example, for the same brocade, if the unit , the value of electrochemical equivalent will be different:
29. 35g/26. 8A•h=l. 095g/(A•h)
For the convenience of readers, the electrochemical equivalents of commonly used metal elements are listed in Table 1-3.