The constant current method is to control the current density of the electrode under test to keep them constant at different values , and then measure the potential value corresponding to each constant current density. After recording the measured potential values of this series, mark one-to-one corresponding points with the current density in the plane coordinate system, and the curve formed by connecting these points is the polarization curve.
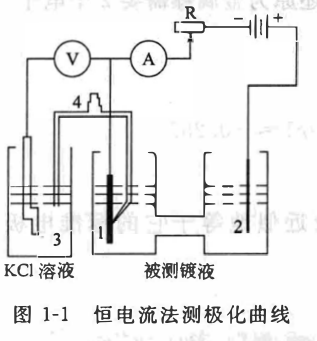
The polarization curve measured by the galvanostatic method reflects that the electrode potential is a function of the current density. The constant current method is relatively easy to operate and is a commonly used method for measuring polarization curves. The equipment and method for measuring the polarization curve by constant current method are shown in Figure 1-1
Put the measured plating solution in the H-shaped electrolytic tank, and the researched electrode (cathode) 1 and auxiliary electrode (anode) 2 are respectively placed at both ends of the H-shaped electrolytic tank . In order to maintain the constant current in the circuit, the resistance is much greater than the resistance of the H electrolyzer (more than 100 times). Adjust R to make the value on the ammeter A constant in turn, and the corresponding electrode electromotive force can be measured from the potentiometer V in turn.
Since the potential value of the reference electrode 3 is known, the electrode potential of the electrode to be tested under different currents . In order to eliminate the influence , the tip of the capillary of the salt bridge 4 should be as close as possible to the surface of the electrode 1 to be tested. The reason why the reference electrode is not directly put into the measured electrolyte is to eliminate the influence . The reference electrode is usually placed in KCl solution. Sometimes an electrolytic cell containing the plating solution to be measured is added between the two electrolytic cells, and a salt bridge is added to make the reference electrode potential less affected.
