Today, I want to tell you about all the different methods of measuring density that we know about, and what they can - or can't - do for us.
Density measurement has a long history - it actually goes back 2200 to the time of King Hiron II of Syracuse. In short, King Shiron II has a crown made of solid gold. Goldsmiths were blamed for adding some silver to the crown. Archimedes was a great mathematician, physicist, engineer, inventor and astronomer who was asked to prove - or debunk - that Hieron's crown was only gold, without destroying it.
Archimedes went home to think about this problem. Having exhausted all his energy to think about this difficult task, he took a shower. (Okay, this part of the story is already made, but read on to see what he finds.) As he sinks into a very full tub, it begins to overflow. He realized that the spilled water was directly related to the immersion of the body. We all know what he did next: he ran across town - naked - yelling: 'Eureka! ' [I found it! ].
In order to verify the gold content of King Hieron's crown, he took the crown and a pure gold bar of exactly the same weight as the crown. He submerges two objects in a container of water. The crown has moved more water than the gold bar, and the water has risen higher - so the crown obviously has more volume. Same mass, but more volume - meaning lower average density. The crown is less dense than pure gold, so it needs to be stretched with another metal.
This experiment helped Archimedes discover the relationship between mass and volume. This leads to some major issues we need to solve when working with gases, solids and liquids:
Do I have pure components? For example: Is pure water used in the production of infusion solutions?
Do I have the correct relationship/focus of the two components? For example: Do soft drinks contain the right amount of sugar?
Am I getting the correct components? For example: is the correct fuel being unloaded from the tank truck?
Which density measurement methods are available?
The enormous diversity of density measurement applications requires better ways to determine mass per volume (or density) to measure density faster, with greater precision, with less sample volume or other than "room temperature" Limited temperature.
Some density measurement methods determine apparent density (weight per volume), while others measure true density (mass per volume).
Note that weight is affected by buoyancy compared to mass.
hygrometer
Hydrometers are based on the relationship between buoyancy and density. It is a floating glass body with a cylindrical rod and a bulb filled with metal weights. Depending on how deep the sample has sunk, the density can be read directly from the upper scale. The deeper it sinks, the less dense the material becomes. If we dip a hydrometer into a glass of water, it will be deeper than a glass of syrup, where it will float higher.

Hygrometers are fast, simple and affordable. They break easily and require large samples. Due to the small size of the hydrometer, the results can easily be misinterpreted. Thermostats are very complex (but need to measure at a specific temperature).
Pycnometer
A pycnometer is a glass bottle of known weight. To determine its exact volume, it is filled with a calibration liquid of known density (such as water). By weighing the water content, the exact volume can be calculated, since the density of water is a known factor. In the third step, a simple calculation provides information about the exact volume (volume = weight/density). When later determining the unknown density of the sample, simply use the same formula (density = weight/volume) in a different way.
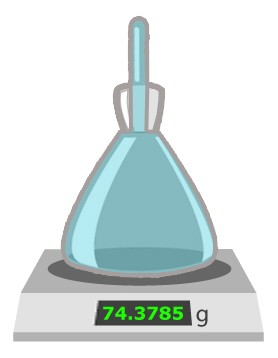
Compared to other methods of density measurement, pycnometers are inexpensive and highly accurate. Unfortunately, these instruments are also prone to breakage, such as hydrometers. In addition to requiring large sample volumes and skilled operators for this rather complex procedure, the method is very slow and time-consuming. Constant temperature is quite a challenge and requires highly accurate balancing for reliable results.
hydrostatic balance
A hydrostatic balance is a very accurate balance in which the volume of a sinker (such as a sphere) connected to one of the dials is exactly known.
The sinker is submerged in the sample and the apparent weight loss of the sinker is determined - actually weighing it. Considering the discoveries of Archimedes and his bathtub, the apparent weight loss of the sinker (Archimedes stepped into the bathtub) is equal to the weight of the liquid it displaces (the water flowing through the bathtub). Therefore, the exact volume and the exact weight are known.
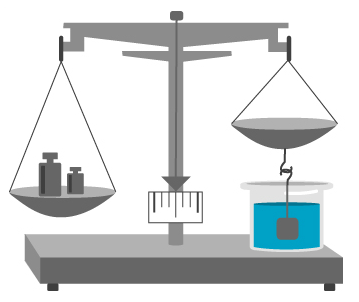
Hydrostatic balancing is highly reliable and accurate. However, all the necessary machinery (such as A/C) is larger than a human operator and - understandably - expensive. Installing one and taking a long time to measure is quite a challenge. Temperature control/insulation can only be established through highly sophisticated air conditioning.
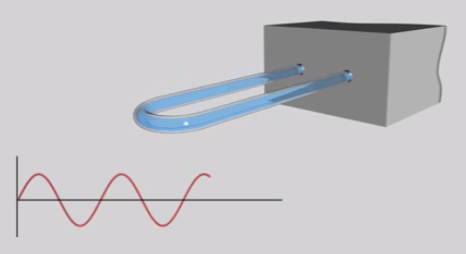
digital density meter
Modern digital density meters - based on the U-tube principle - measure the inertial mass of a known sample volume. The sample is filled into a U-shaped tube mounted on a balance weight. Then the U-tube is excited and starts to oscillate. Depending on the filled sample, the characteristic frequency of the oscillating tube is different. Measure the change in frequency and determine the density. So high frequency may be related to low density and vice versa.