overview
Plasmas of gas make up 99 percent of our universe and are found mostly in stars. Although rare on Earth, natural plasmas include lightning, the northern lights, and St. Elmo's fire. Table 40.1 lists certain plasmas and characterizes them by particle density and temperature.
Plasmas can be produced and controlled by ionizing a gas with an electromagnetic field of sufficient power. A useful form of gas plasma is created by introducing a gas into a reaction chamber, maintaining a pressure between 0.1 and 10 Torr, and then applying radio frequency (rf) energy. Once ionized, the excited gas reacts with the surface of the material placed in the glow discharge.
The physical and chemical properties of a plasma depend on many variables; chemistry, flow, distribution, temperature and gas pressure. In addition, RF excitation frequency, power level, reactor geometry and electrode design are equally important. When the power to the plasma is turned off, the disintegrated gas molecules quickly recombine into their natural state.
type of plasma
Plasmas occur over a wide range of temperatures and pressures, however, all plasmas have approximately equal concentrations of positive and negative charge carriers such that their net space charge approaches zero.
In general, all plasmas fall into one of three classifications. High-pressure plasma elements, also known as thermal plasmas, are in thermal equilibrium (typically energy > 10,000°C). Example 1 shown in Table 40.1 includes stellar interiors and thermonuclear plasmas. Hybrid plasmas have high temperature electrons in mesophilic gases (~100 to 1000°C) and form at atmospheric pressure. Arc welders and corona surface treatment systems use hybrid plasmas. The cold plasmas that are the focus of this chapter are not in thermal equilibrium. The temperature (kinetic energy) of free electrons in an ionized gas can be 10 to 100 times higher (up to 10,000°C) when the bulk gas is at room temperature, creating an unusual, very chemically reactive ambient temperature.

There are two types of cold plasmas, determined by the electrode configuration. The primary plasma is generated directly by RF energy between the chamber electrodes. Secondary plasma exists downstream of the energy field, carried by gas flow and diffusion. Secondary plasmas are less desirable for surface modification because the plasma reacts less the farther downstream the rf field is from the part to be treated. One part may shield the other, creating inhomogeneity and less surface area to treat before all active material is locally depleted, reducing effectiveness at larger loads.
Typical Plasma Treatment Cycle
Modern plasma treatment equipment is fully automated. A typical continuous processing plant is shown in Figure 40.1.
To prepare for treatment, process engineers enter setpoints into the microprocessor controller. Conditions for this procedure have previously been determined to be valid for a particular application. A key switch locks the set point so the operator cannot inadvertently modify the process. A process can consist of single or multiple process steps, each step comprising a complete plasma cycle.
When loading the chamber, the machine operator places the parts on the electrodes/shelves. After closing the door, the operator starts the process by pressing the start button. The controller monitors and controls the process until completion.
The first step taken by the controller is to evacuate the reaction chamber by opening the isolation valve of the Vacuum Pump. Process gas enters the chamber at a predetermined base pressure. The delay allows the pressure in the reaction chamber to stabilize, and then the power is turned on to generate the plasma. The controller starts a process timer. The impedance matching network continuously and automatically minimizes the power mismatch between the generator and the chamber as long as the RF power is on.
This step ends at the end of the process time or after reaching an optional temperature set point, at which point the RF power and process gases are turned off. A Vacuum Pump evacuates process gases and by-products from the chamber, and the system repeats the entire cycle for the next step. If the last step has been completed, the chamber is vented to atmosphere and the controller alerts the operator that the process is complete.
plasma chemistry
Three properties of cold plasmas—chemical dissociation, ion acceleration kinetics, and photochemistry—make this unique environment effective for surface treatment.
Exposing the gas to sufficient electromagnetic energy causes it to break down, forming a chemically reactive gas that rapidly modifies the exposed surface. At the atomic level, plasmas contain many different energy levels of ions, electrons, and various neutral species. One of the excited species formed is free radicals, which can directly react with the surface of organic materials, leading to significant changes in their chemical structures and properties. Modification sites also occur when ions and electrons bombarding a surface gain enough kinetic energy from a changing electromagnetic field to knock atoms or groups of atoms from the surface. In addition, gas phase collisions transfer energy - forming more free radicals, atoms and ions.
The dissociated combination of matter emits photons as they return to the ground state. The spectrum of this glow discharge includes high-energy UV photons that will be absorbed into the top surface of the substrate, creating more active sites. The color of the glow discharge depends on the chemistry of the plasma, and its intensity depends on process variables.
The plasma process alters only a few molecular layers, so appearance and bulk properties are usually unaffected. In addition, the plasma changes the molecular weight of the surface layer by cutting (reducing the molecular length), branching and crosslinking the organic material. The chemistry of the plasma determines its effect on the polymer.

Surface treatment method
Surface treatment is applicable to many polymer processes and plasma is a chemical process. Three types of cold plasma treatments are used in treating polymers:
1. Activation of the plasma uses a gas or gases that react with the product to change its chemical properties. Such plasmas use oxygen, ammonia, air, halogens, and other gases to clean surface contaminants, microbond surfaces, and replace various chemical groups onto polymer chains.
Activation of the plasma is discussed below.
2. Grafted plasma treatment first activates the surface by exposing it to a chemically inert plasma, and then immerses the surface in the vapor of an unsaturated monomer (without generating a plasma). Free radicals previously formed on the polymer surface initiate the grafting reaction with the reactive monomer.
3. Plasma polymerization uses plasma energy to initiate a gas-phase polymerization reaction, resulting in the deposition of organics on surfaces within the plasma chamber.
plasma activated plastic
The activated plasma has three competing molecular reactions that can simultaneously alter the plastic. The respective ranges depend on chemical and process variables. They are as follows:
1. Ablation (microetching), or removal or removal of surface topography by evaporating surface material
2. Cross-linking, or creating covalent bonds or links between parallel long-chain molecular chains
Substitution, replacing atoms in the molecule with atoms in the plasma
Ablation is an evaporation reaction in which the plasma breaks the carbon-carbon bonds of hydrocarbon polymers. As the molecules get shorter, their volatile monomers or oligomers boil (melt), and they are expelled. Ablation is important for surface cleaning and is used for surface etching when required. Cleaning removes external organic contaminants such as hydraulic oil and mold release agents from polymer surfaces. It is also important to remove internal contaminants such as processing aids and internal lubricants that have lodged on the surface. Typically, oxygen-containing plasmas are chosen to facilitate rapid breakdown of suspected contaminants into volatile by-products.
Plasma cleaning is more effective than cleaning by steam degreasing or other methods. Plasma produces an "ultra-clean" surface; however if severe contamination is present, parts may be pre-cleaned by ultrasonic cleaning or solvent-vapor degreasing, keeping plasma treatment time to a minimum and thus maintaining cost-effectiveness.
一旦清洗完毕,等离子体开始烧蚀聚合物的顶部分子层。非晶态,填充态和结晶态部分将以不同的速率去除,从而提供有效增加表面形貌的技术,以增加机械粘附力或去除在成型过程中形成的弱边界层。
另一方面,交联用无氧惰性气体(氩气或氦气)完成。等离子体产生表面自由基后,它们与邻接分子或分子片段上的基团反应形成交联。该工艺增加了表面的强度,耐温性和耐溶剂性。
与消融或交联不同,取代用血浆中的活性物质代替表面上的一个原子或基团。在这种情况下,表面上的自由基位点可以自由地与等离子体中的物质反应,包括但不限于自由基,从而通过添加共价键合的官能团来改变表面化学性质。处理气体的选择决定了将在改性聚合物上形成哪些基团。用于聚合物等离子体处理的气体或气体混合物包括氮气,氩气,氧气,一氧化二氮,氦气,四氟甲烷,水和氨。每种气体产生独特的等离子体化学。通过等离子体诱导的氧化,硝化,水解或胺化可以快速增加表面能。
非常积极的等离子体可以从相对良性的气体中产生。例如,氧和四氟甲烷(氟利昂14)等离子体含有氟的自由基。已知氟自由基的氧化与更强的无机酸蚀刻剂溶液的氧化同样有效,其中一个重要区别是:不使用有害和腐蚀性材料。一旦关闭等离子体,激发的物种重新组合成它们最初的稳定和非反应形式。在大多数情况下,不需要处理废气排放物。
含有氧气的气体通常在增加表面能方面更有效。例如,聚丙烯的等离子体氧化在几秒钟内使初始表面能量增加29达因/厘米,远超过73达因/厘米。在73达因/厘米时,聚丙烯表面完全是水可润湿的。
增加的表面能导致产生极性基团的等离子体,例如羧基,羟基,氢过氧基和氨基。更高能量(亲水)表面转化为更好的润湿性和改性表面对粘合剂,油漆,油墨和沉积的金属膜的更大化学反应性,从而提供改进的粘合性和长久性。
Characterization of enhanced surface reactivity in the laboratory by studying water wettability. Wettability describes the ability to spread and penetrate a surface; it is measured by the contact angle between a liquid and a surface. The relationship between contact angle and surface energy is inverse - contact angle decreases as surface energy increases. Wetting can be easily produced on normally non-wetting materials such as polyolefins, engineering thermoplastics, fluoropolymers, thermosets, rubbers and fluoroelastomers.
Inert gases (argon, helium, etc.) generate surface radicals that, when reacting with other radicals on the surface, create a molecular weight change, or react with air to remove the part from the chamber , thereby increasing the surface energy.
Process gases such as fluorocarbons are typically lower energy or hydrophobic surfaces by substituting abstract hydrogens with fluorine or trifluoromethyl groups to form fluorocarbon surfaces. Fluorination is advantageous in some medical applications where wetting the catheter with blood is undesirable. A non-wettable barrier also inhibits chemical penetration, which is important for specialty packaging.
Adhesion
Bonding during manufacturing is an area of expertise, but in general, cleanliness and wetting are required for good bonding.
High surface energy alone does not guarantee better adhesion; however, the versatility of the process allows tuning the surface chemistry for either great adhesion or great product performance. It is not uncommon for the focus of failure to shift from the bond line to the adhesive or into the adhesive, which multiplies many times over. Table 40.2 and Table 40.3 list typical examples of plasma modification for epoxy bonded materials3,4 and coating5 material ranges.
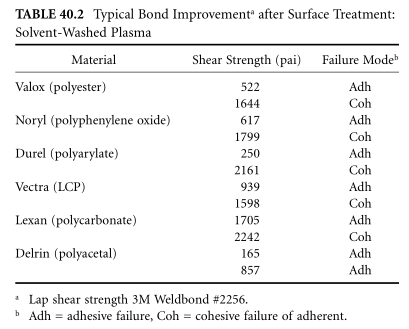
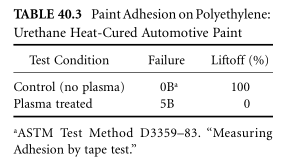
Very reactive polymers, such as elastomers, have shelf lives measured in minutes or hours. Flexible molecular chains transform high-energy functional groups into bulky ones. Once the active surface is treated and the adhesive is properly applied, the finish adheres permanently to the surface. Thus, priming of the treated elastomer fixes the surface chemistry.
Summarize
Plasma surface treatment is an effective method for modifying the surface of various polymers and elastomers. The bond strength of the treated material generally exceeds that of the adhesive. The plasma process is not operator sensitive; other important characteristics include reproducibility, cleanliness, and the ability to deliver high reliability bonds more consistently.






