The surface of the reticulated polymer substrate is subjected to elemental fluorine-attenuation with an inert gas continuously for a short period of time in a suitable reaction chamber. Thus, the surface energy of the polymeric material is increased to such an extent that excellent adhesion to other polymers such as paints and adhesives is achieved.
This fluorination technique is feasible on an industrial scale due to the amazing technological developments in chemistry over the past 20 years, which have made fluorine not only an important starting substance but even an integral part of large-scale technical operations.
fluorine
Fluorine, an almost colorless gas, is one of the stronger oxidizing agents; it is surpassed only by a few other oxidizing agents (e.g. chlorine fluoride, chlorine trifluoride). It reacts with almost all organic and inorganic substances; firstly, nitrogen and the noble gases helium, neon and argon, and some metal fluorides in the highest valence state and other fully fluorinated compounds (such as CF4 or SF 6) A few exceptions are included. The enormous reactivity of fluorine can be explained by the low dissociation energy of the molecule itself and the very strong fluorine bond-forming interactions with other atoms. Furthermore, since the fluorine atoms are rather small, the spatial relationships in fluorine compounds admit of high coordination numbers of the relevant central atoms.
The extreme aggressiveness of fluorine has long limited its use in industrial applications. In fact, until 1936, the Technical Encyclopedia states: "Owing to the difficulties of manufacture and storage, fluorine is of no practical interest to industry." Only a little more than two decades ago, all the difficulties that have weakened fluorine on a large scale can be considered to have been overcome.
Currently, primary fluorine in liquefied state is even transported in fuel trucks. It is primarily used to make highly volatile UF6, which is known to separate the uranium isotopes U 235 and U 238. Therefore, fluorine has become a key product of the nuclear industry. In Germany, Calichem AG, which has always been a specialist in the field of fluorine chemistry, is a manufacturer of very reactive substances.
Continuous Surface Fluorination Plant
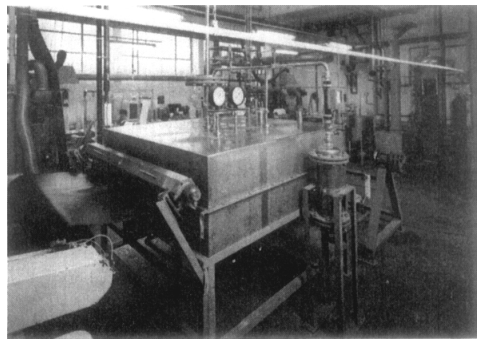
Figure 41.1 illustrates a block diagram of an apparatus for pretreating mesh material with fluorine. 4 Figure 41.2 shows a practical setup. The fluorine supplied from steel pressure cylinders is attenuated to a certain concentration with inert gases (nitrogen, noble gases and compressed air) in the corresponding dosing devices. This fluorine-inert gas compound is available to the system via line 9 in Figure 41.1. The attenuation step can be eliminated by instead connecting to a steel pressure bottle filled with fluorine-nitrogen compound.
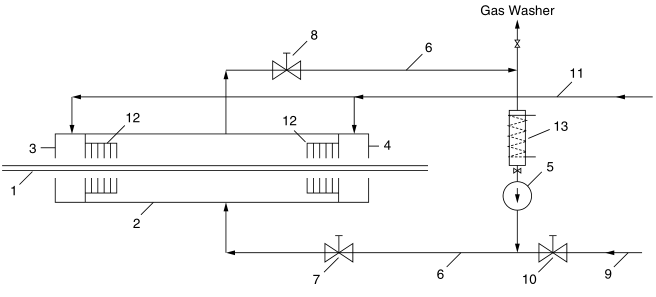
Due to the higher fill pressure, the 10 vol% standard compound delivered 50% of the fluorine delivery obtained using the pressure bottle containing the basic fluorine. After filling the reaction chamber (2: Figure 41.1) with weakened fluorine, the fluorine-inert gas compound is circulated in the reaction chamber 2 by means of the pump (5) through the line 6 and the two shut-off valves (7 and 8).
The mesh material to be pretreated enters the chamber 2 through the inlet lock (3) and exits through the outlet lock (4).
The fluorine contact time and fluorine concentration affect the surface energy of the polymer. The achievable surface effects also depend on the chemical quality of the polymer.
Due to the extreme reactivity of elemental fluorine, all polymeric materials that can be treated to eliminate hydrogen atoms can in principle be activated. Gas locks 3 and 4 are supplied with inert gas through pipe 11 . The airlock, together with the flow resistance (12) connected in series, largely prevents fluorine and hydrogen fluoride (HF) from escaping into the surrounding air. Such an airlock system is described in detail in Ref. From pipe 9 and via dosing valve 10, the escaped portion of fluorine is replaced, but a small amount of fluorine is lost to HF due to chemical conversion with reactants. Since partial fluorination causes surface activation, which is sufficient for many purposes, the amount of fluorine actually consumed or the amount of HF produced is relatively small.
The low portion of HF in the system is removed by the hydrogen fluoride absorber (13 in Figure 41.1). This cleaning is also necessary if the fluorine is dosed by the glass rotameter. The absorber consists of a tube (diameter, 50mm; length, 450mm) made of monel, nickel or steel and filled with granular porous sodium fluoride. Welded to the sides of the pipe are caps with supply and discharge pipes. The absorber can be heated to 300°C by means of an electron tube furnace. HF absorption takes place at room temperature. For regeneration, the absorber is heated to + 300°C in a nitrogen stream. Suitable porous sodium fluoride can be produced by heating granular or granulated sodium difluoride to 250-300°C in a nitrogen stream.
If necessary, the whole system can be cleaned by a gas cleaning machine: the F2 and HF parts in the flushing gas are absorbed by the countercurrent diluted potassium lye and are harmless. The exhaust is completely free of pollutants.
Safety Instructions
The hazards of gaseous fluorine and hydrogen fluoride transported into the open air through airlocks are comparable to those caused by the release of ozone, which inevitably occurs due to corona pretreatment. Known and approved safety measures for ozone also apply to fluorine and hydrogen fluoride. For these gases, the limit is 0.1 ppm in air, such as ozone.
Bioassays have shown that fluorine and hydrogen fluoride are many times less toxic than ozone.
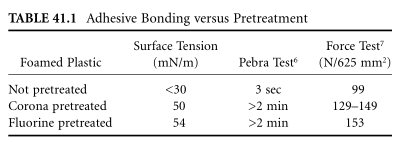
Because fluoride tastes like chlorinated water, poisoning is very rare even in low concentrations.
If the critical content of fluorine entering the air is exceeded, a chemical Detector is installed to give an audible alarm and interrupt the supply of fluorine to ensure on-the-job safety.

