The thickness of the electrode film has a great influence on the utilization rate of the active material of the battery. When the thickness of the negative electrode film is constant (77.5 μm) and the thickness of the positive electrode film is 107.5 μm, 92.5 μm and 85 μm, the first cycle of the positive electrode active material The utilization rate was significantly improved, being 108.9 mAh/g, 130.2 mAh/g, and 132.1 mAh/g, respectively. It can be seen that a thinner positive electrode film is beneficial to improve the utilization rate of active materials. The thickness of the positive electrode film selected in the later test cell is
80 µm (the capacity of the positive active material can reach 140 mAh/g), and the film thickness of the negative electrode is 67.5 µm.
Positive film thickness is too large
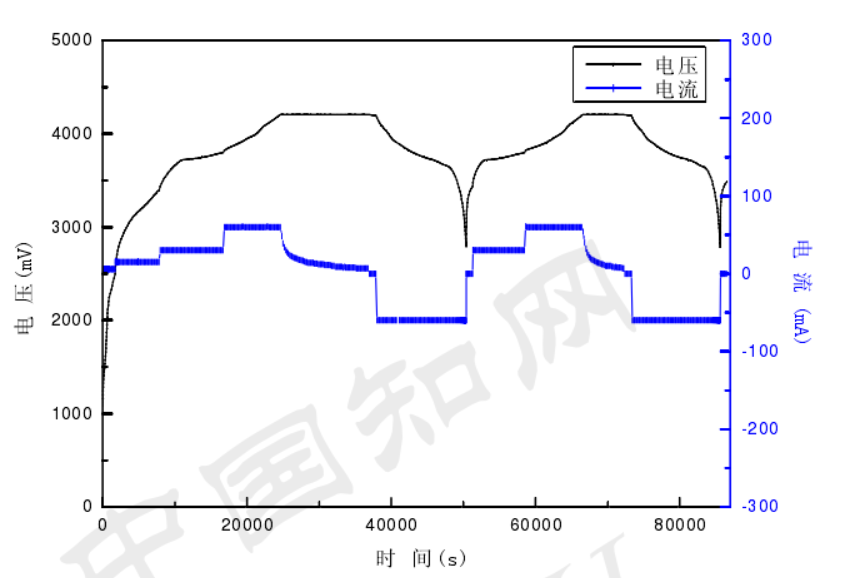
The test results show that for a battery with an excessively large positive electrode film, the constant current charging time is relatively short, while the constant voltage charging time is relatively long during the charging process, resulting in low charging efficiency. At the same time, due to the long migration distance of ions in the electrode film, the concentration polarization discharge capacity is low, resulting in low utilization of active materials in the battery. Figure 3-1 shows that it takes a long time to charge the battery for the first time. Table 3-4 lists the charge and discharge data of the first two cycles of the battery. The charge and discharge efficiency of the second cycle is relatively low.
92.2% and the discharge capacity has declined, which are all related to the excessive thickness of the positive electrode film.