This paper discusses and analyzes the coating defects caused by surface tension, and proposes relevant preventive measures to provide some reference experience for relevant staff.
1 Overview
Discuss coating defects caused by surface tension, many of which are closely related to surface tension, such as convection nests, also known as be nard cells, cratering, thick edges, retraction or dewetting, bond breakage or delamination, etc. The text mainly explains the basic knowledge related to surface tension, analyzes and discusses the causes of these ills and preventive measures.
2 Basic knowledge about surface tension
2.1 Surface energy and surface tension
From daily life, it is known that it takes work to make a system form a new surface, or to give the system a certain amount of energy, such as blowing up soap bubbles, splitting wood, etc. This indicates that the surface has more energy than the body. In equilibrium, the increase in energy per unit surface area is the surface energy. For liquid liquids that are fluid, it can be considered to be always in equilibrium and can also be expressed in terms of the force acting on a unit length which is known as surface tension. The unit of S I of surface energy is m J/m2. The unit of S I of surface tension is m N/m. Surface energy and surface tension have the same dimension and value, and are often represented by the Greek letter γ.
If a system is composed of a certain amount, its surface tension is constant at a certain temperature and pressure, and if the surface area ΔA is increased, the increase in the energy of the system ΔG is: ΔG = γ·ΔA (1)
It can be seen that any system tends to be in the lowest energy state, such as water flowing to a lower place. Eq. (1) shows that in order to reduce the energy of the system, liquids always tend to minimize the surface area ΔA. Among the various morphologies, the spherical surface area is the smallest, so small droplets (which can be ignored by gravity) always tend to form spherical.
Another way to reduce the energy of the system is to reduce the surface tension. As a result, the surface of the object will always absorb some substances with low surface tension, such as oil stains. In multi-component solutions, substances with low surface tension always tend to be enriched on the surface to reduce the surface tension of the system.
There are many methods to directly measure the surface tension of liquids, such as drop volume method, ring method, hanging piece method, drop profile method, maximum bubble pressure method, etc.
Liquid surface tension is temperature-dependent. In general, the surface tension of solvents and water for coatings decreases as the temperature rises. Table 1 shows the surface tension values for some liquids at 20 °C.

2.2 The relationship between the pressure difference between the inside and outside of the bending liquid level and the surface tension

In Eq. (3), R1 and R2 are the primary radii of the surface.
2.3 Surfactants
In coatings, surfactants are often used as emulsifiers, wetting agents, dispersants, etc.Substances that significantly reduce the surface tension of liquids are called surfactants. Unless otherwise specified, commercially available surfactants are for water, i.e. they can only significantly reduce the surface tension of water. Fluorinated surfactants can reduce the surface tension of both water and organic solvents.
The molecular structure of surfactants is characterized by "amphiphilicity", that is, one part of the molecule is hydrophilic and the other part is lipophilic. The lipophilic part is generally a long-chain hydrocarbon group (fluorosurfactants are either fluorocarbonic or partially replaced by fluorine). Depending on whether the hydrophilic group is ionized in water or not, surfactants can be divided into ionic and non-ionic types. In ionic surfactants, they are divided into anionic surfactants, cationic surfactants and amphoteric surfactants according to the lipophilic groups that are connected to anions, cations and zwitterionic ions. The surfactants used in practice are often various types of composite types.
Here's a brief explanation of why surfactants can reduce the surface tension of water. From Table 1, it can be seen that the surface tension of hydrocarbon group is generally about 20 ~30 m N/m, while the surface tension of water is 72 .8 mN/m. When the surfactant is dissolved in water, it will be enriched on the surface of the water, with the hydrophilic group extending into the water and the lipophilic group facing the air. When the surface of the water is covered by lipophilic groups, the surface tension is mainly determined by the hydrocarbon group, which causes the surface tension to decrease significantly. 2.4 The conditions under which the coating liquid spreads on a solid matrix are discussed above and only the surface tension or surface energy is concerned. The interface between a liquid or solid and air is called a surface. The knowledge of surface tension and surface energy applies to all interfaces, such as solid-liquid interfaces, where interfacial energy or interfacial tension exists.
When a liquid is dropped on a solid surface, a droplet is often formed to stay on the solid surface, as shown in Figure 1. At the junction of solid (S), liquid (L) and gas (G), the angle from the solid/liquid interface through the liquid to the gas/liquid interface is called the contact angle, which is represented by θ. From the perspective of force balance, the equilibrium contact angle has the following relationships with the solid/gas interfacial tension (γSG), solid/liquid interfacial tension (γSL), and liquid/gas interfacial tension (γLG):
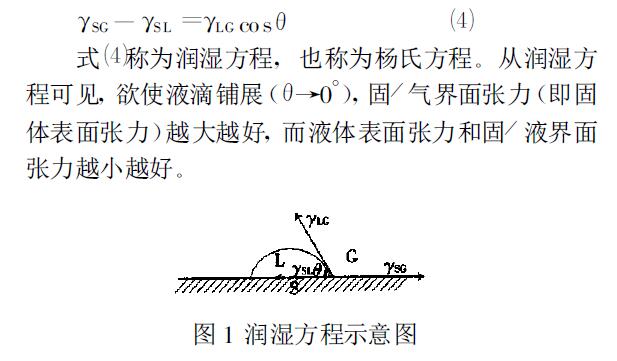
As can be seen from the wetting equation above, solids with high surface energy (or high surface tension) are more likely to be wetted than solids with low surface energy. The surface energy of solids is difficult to determine. Solid surfaces are divided into two main categories based on whether they are easily wetted: high-energy surfaces and low-energy surfaces. The surface tension of typical liquids is below 100 m N/m. Therefore, this value is used as the dividing value between high-energy and low-energy surfaces. Metals and their oxides, sulfides and inorganic salts are high-energy surfaces, which are easily wetted by ordinary liquids. The surface energy of polymers or solid organic compounds is similar to that of ordinary liquids, and their wettability varies with the composition and properties of the solid-liquid phases in contact with each other.
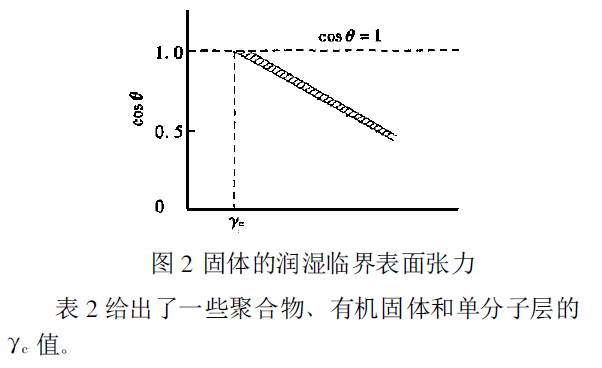
When droplets of liquids with different surface tensions are on the same surface of a low-energy solid, the contact angle should decrease as the surface tension of the liquid decreases (CO Sθ increases). The test results show that taking the surface tension of the liquid as the abscissa and cos θ as the ordinate, a narrow band as shown in Figure 2 is obtained, and the surface tension of the liquid corresponding to the intersection of the lower limit of the narrow band and the intersection of cos θ=1 is called the wetting critical surface tension of the solid, which is expressed by γc. From this, the meaning of γc is that only liquids with a surface tension less than this value can be spread on this solid.
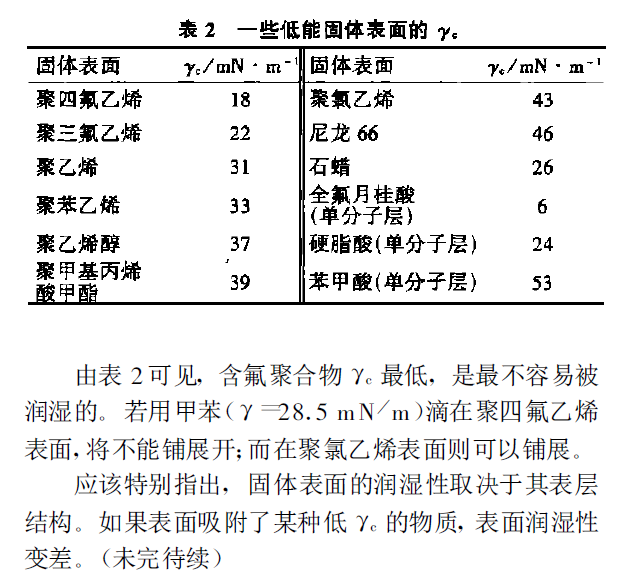
This paper summarizes the coating defects caused by surface tension and the prevention and control methods.
Gong Haiqing's "Coating Defects Caused by Surface Tension 1"
