Adhesion refers to the mutual attraction between the contact parts of two different substances. Several important factors affecting the adhesion of coatings are introduced below.
1. Roughness
If the surface to be coated has a certain roughness, the contact area with the coating can be increased to obtain better adhesion, but the coating must be able to completely wet and effectively penetrate and cover the rough surface to be coated, otherwise, water will When it penetrates the coating and reaches the uncovered part of the coating, it accumulates there, causing metal corrosion.
2. Wetting
The coating must first wet the surface to be coated to form adhesion, otherwise, there will be no molecular level contact between the coated surface and the coating, there will be no interaction, and no contribution to the adhesion of the paint film. Wetting requires the coating to have a lower surface tension than the surface being coated. But the north and south tides remind you: good wetting does not guarantee good adhesion of the paint film.
It is more complicated when coatings with certain additives (containing single polar groups and long hydrocarbon chains) are used directly on steel. The principle of influence of this type of additive (containing a single polar group and a long hydrocarbon chain) is illustrated with n-octanol. Put n-octanol on the surface of a clean steel plate, the surface tension of n-octanol is lower than that of the steel plate, and spontaneously form layers on the steel plate. However, if n-octanol is coated on a steel plate to form a film, it will be found that n-octanol shrinks into droplets on the surface of the steel plate. This is because the low surface tension of n-octanol is caused by its linear hydrocarbon chain. After it spreads on the polar surface of the steel plate, the hydroxyl group is in contact with the surface of the steel plate and is adsorbed, while the linear hydrocarbon chain The chains are stretched in the air on the surface of the steel plate, forming a fat warp surface. This surface has a lower surface tension than n-octanol. Therefore, n-octanol does not wet the surface of the fat burner and shrinks into droplets. In the same way, when dodecylbenzenesulfonic acid is used as a catalyst for coatings, it will lead to poor adhesion to steel plates, and the adhesion of latex coatings is also affected by the surfactant layer between the coating and the surface to be coated. These influencing factors should be avoided in coatings. For example, the catalyst commonly used in coatings is p-toluenesulfonic acid.

3. Paint penetration
The behavior of paint penetrating into the pores of the coated surface is similar to the behavior of liquid penetrating into capillaries. Here, the factors affecting infiltration are analyzed with the help of the capillary formula. In the time t (s), the length of the liquid entering the capillary with a radius of r (cm) is defined as the penetration value L (cm), γ is the surface tension of the liquid (mN/m), θ is the contact angle, and η is the viscosity ( Pa·s):
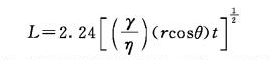
Here, the capillary radius can be compared to the scale of the micropore gap of the coated surface, that is, the roughness of the coated surface. Factors that increase the penetration value L can increase the degree of liquid penetration into pores and crevices. When the contact angle θ is 0, its cosine is 1, and L is large at this time. When the surface tension of the coating is smaller than that of the surface to be coated, the cosine of the contact angle θ can only be 1. High coating surface tension γ also increases L, but γ also affects θ and other properties of the coating, so γ lacks room for adjustment. What can be controlled is the viscosity, which should be as low as possible.
The pigment particles in the coating are generally larger than the size of the micropores and gaps, and cannot enter the micropores and gaps of the coated surface. Therefore, the viscosity of the continuous (outer) phase of the coating (the viscosity of the base material) plays a decisive role. rather than the overall viscosity of the paint. The lower the viscosity of the continuous (external) phase, the faster the penetration.

After construction, the viscosity of the base material increases with the volatilization of the solvent, and the wet paint film must maintain a low viscosity for a long enough time to be able to penetrate effectively. The viscosity of the resin solution increases with the increase of the molecular weight, and the lower molecular weight resin can penetrate effectively due to its low viscosity and endow the coating with excellent adhesion. Baked coatings generally have better adhesion than coatings formed by drying at room temperature. As the temperature rises in the oven, the viscosity of the outer phase of the coating decreases, increasing penetration into irregularities of the coated surface.
Paints with low viscosity, slow solvent evaporation and low crosslinking rates, baked coatings generally have better adhesion.
In short, in order to make the paint film have good adhesion, the surface of the substrate is required to have a certain roughness, and the paint must be able to fully wet and penetrate into the micropores and crevices on the surface of the substrate.