Chloride ion intrusion is one of the main reasons for the corrosion of steel bars in concrete. Chloride ions can destroy the passivation film on the steel surface, cause local corrosion of the steel, and have a catalytic effect on the corrosion process. The development of the modern construction industry needs to quickly and accurately measure the chloride ion content in building materials, so as to realize the effective evaluation of the durability of building materials and the effective protection and timely repair of steel corrosion.
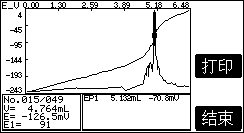
1. Determination example of chloride ion in cement
1. Sample processing
Weigh about 5g sample, accurate to 0.0001g, put it in a 250mL beaker, add 20mL water, stir to disperse the sample completely, then add 25mL nitric acid (1+1) under stirring, add water to dilute to 100mL, add 2mL Chloride ion standard solution and 2mL hydrogen peroxide, cover with a watch glass, heat to boil, slightly boil for 1min-2min. Cool to room temperature, rinse the watch glass and glass rod with water, remove the glass rod, and add a magnetic stir bar.
2. Sample analysis
Connect PCl-1-01 chloride ion electrode and 217-01 double salt bridge reference electrode (add saturated potassium nitrate to the outer salt bridge), place the beaker on the automatic titrator, and titrate . At the same time do a blank test.
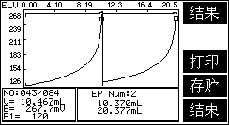
2. Example of determination of chloride ion in concrete admixture
1. Sample handling
Accurately weigh 5.0000g of admixture sample, put it into a beaker, add 200ml of water and 4ml of nitric acid (1+1) to make the solution acidic, stir until it is completely dissolved, if it cannot be completely dissolved, filter it with rapid quantitative filter paper, and use Wash the residue with distilled water until there is no chloride ion.
2. Sample titration
Connect the PCl-1-01 chloride ion electrode and the 217-01 double salt bridge reference electrode ( add ), place the beaker on the automatic titrator, add the sample, and pipette 10ml 0.1mol /L sodium chloride standard solution, titrate with 0.1mol/L silver nitrate standard solution, record the end point volume V1, then add 10ml 0.1mol/L sodium chloride standard solution, continue to titrate with silver nitrate standard solution, record the end point volume V2. At the same time do a blank test.
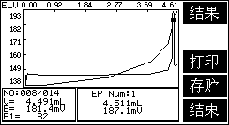
3. Example of Chloride Ion Determination in Concrete
1. Sample handling
Weigh 5g of finely ground concrete powder, place it in a 250ml Erlenmeyer flask, add 250.0ml of water, shake and soak for 6h, and filter with rapid quantitative filter paper.
2. Sample titration
Connect the 216-01 silver ion electrode and the 217-01 double-salt bridge reference electrode (add saturated potassium nitrate to the outer salt bridge), place the beaker on the automatic titrator, pipette 50ml of filtrate into the beaker, add 2 drops of phenolphthalein, Titrate with nitric acid (1+3) until the red color just fades, then add 10ml of starch solution, titrate with silver nitrate standard solution, and record the volume V. At the same time do a blank test.