Solventborne formulations have a naturally low surface tension, wet out easily and transfer well to most substrates, even though they may have a higher spread coefficient on selected substrates. There is an increasing shift to water-based inks due to environmental concerns, and there are inherent issues with surface wetting, foaming, flow and leveling that are common to all water-based systems. Water-based inks require alcohols and surfactants to lower their surface tension to acceptable levels for transfer, spreading and adhesion.
Alcohols have low surface tension and are "inactive," but most common surfactants used in aqueous systems generally have high surface activity, which varies with concentration, molecular weight, and structure. Coating application is a dynamic process and reactive surfactants cause surface tension to vary with speed and formulation. Surfactant activity directly affects coating spreading and adhesion. The surface tension of the ink needs to be lower than the wetting tension of the substrate for good printability, adhesion or ink spreadability.
Recipe Variations
The formulation and application of water-based inks are influenced to some extent by controllable external factors, such as transportation and storage, freezing and thawing, before and after each process takes place. The physics of handling and mixing systems are largely fixed and uncontrollable. Many facilities are subject to the uncontrollable effects of seasonal changes—hot summers versus cold winter environments, for example. Inks can, and often do, change in the field of formulation, and more so before and during use. What is often formulated and tested in the lab is not what is ultimately used in the application process. Alcohol and reagents are lost through evaporation and these losses may go undetected unless testing can be done at the formulation and processing site.
When any ink or coating is applied, the surfactant molecules in the bulk solution react by diffusing to the interface. It is at this interface that the surfactant molecules try to align with their hydrophobic ends pointing towards this interface, thereby lowering the surface tension. The concentration and nature of the surfactant determines how much the surface tension can be lowered, but the rate at which diffusion occurs affects the rate at which wetting occurs. Wettability and ultimately "paintability" are influenced by a combination of the physical and chemical properties of the ingredients. This article will examine the field of controllable factors, focusing on techniques for measuring the dynamic surface tension of inks and the wetting tension of solid surfaces (substrates) to be coated.

Figure 1 / Dynamic (CMC) determination
Surface Tension and Surfactants
Surface tension can be thought of as the force that, within its own bounds, "holds" a fluid together in the presence of air—the tangential intermolecular attraction between adjacent molecules. Surface tension determines whether a coating will wet and spread on or retract from a solid surface. Surface tension is expressed as force per unit width; dyne/cm (or mN/m). Water has a high surface tension, in the range of 72 dynes/cm, while alcohol has a lower surface tension of 20 to 22 dynes/cm. Solvents typically used in solvent-based formulations are in the 20–30 dynes/cm range. This is the main reason why it is a major challenge to replace solvent-borne systems with much lower surface tensions by water-based formulations with high intrinsic surface tension values.
Surfactants are used because of their ability to lower surface tension. They are classified by the ionic charge of the interacting portion of the molecule's surface. Anionic surfactants have a negative molecular charge, cationic surfactants have a positive charge, and nonionic surfactants have no charge. Amphoteric compounds have positive and negative charges. Anionic and nonionic surfactants provide most of the industrial surfactant requirements. Surfactant selection is based on specific needs, and often a mixture of surfactants is used.
In general, surfactants with lower molecular mass (lighter) (shorter hydrophobic tail) diffuse faster to the interface and adsorb vertically at the interface, allowing compressive forces to act on the surface, thereby reducing surface energy or surface tension . Nonionic surfactants with oxirane groups generally diffuse to surfaces very quickly, while fluorinated surfactants are slower and more efficient at equilibrium. Most higher concentrations of surfactants create strong molecular attractions between adjacent molecules, resulting in the formation of a strong surface film, the strength of which determines the surface properties of a surfactant solution.
At the instant of deposition of a coating on a substrate, at zero new surface generation time, the concentration of surface-active molecules at the interface will be the same as in the bulk solution; equal to the surface tension of a pure solvent. Surfactant molecules then start to diffuse and adsorb at the newly created fluid/substrate interface and fluid/air interface. It takes a finite amount of time for the surface tension to reach equilibrium, ranging from seconds to minutes. This is why the relevant parameter in the design formulation is dynamic surface tension rather than static (also known as equilibrium) surface tension.
Surface tension determines whether a coating will wet and spread on or retract from a solid substrate. The ink exhibits both adhesion, which is a measure of how well the coating bonds to the substrate, and cohesion, which is a measure of how well the coating adheres to itself. The diffusion coefficient is the difference between the work of adhesion and the work of cohesion. Spontaneous spreading occurs if the work of adhesion is greater than the work of cohesion. If the work of cohesion is greater than the work of adhesion, retraction, a type of surface defect, will occur because the coating will preferentially bond to itself.
The classic view is that at the critical micelle concentration (CMC) of a surfactant, many properties related to surface tension, such as detergency, foaming, and wettability, are either maximized or minimized. However, these relationships are based on classical measurements limited to static surface tension conditions when an equilibrium is established between the surface layer and the bulk solution. Dynamic surface tension measurements of active surfactants reveal higher levels of surfactant effectiveness, which do not necessarily correlate with equilibrium CMC. Dynamic measurements more accurately reflect actual, in-process, surfactant and coating performance. In fact, if you limit the surfactant migration time (by using a faster coating process), you need more surfactant to do the same job as a slower process.
Ink Formulation Variables
Aqueous systems have some inherent advantages over solvent-based systems, such as lower dissolution rates and higher viscosities, but they are more pH sensitive. Common formulations typically combine soluble resins in water-amine-emulsion systems. Therefore, the importance of pH cannot be overemphasized. If it is too high it will burn out the pigment and change the paint viscosity, if it is too low it may "kick" the resin and pigment out of the water and leave a pigment deposit. The solubility problem of water-based ink is mainly due to the lack of a resin system that is completely soluble in water. Some water-based inks require the use of organic co-solvents to achieve low enough surface tension to wet certain substrates.
Pigments have a range of properties that affect bond strength, adhesion, pH stability, viscosity, color and coatability. Important properties of binders include the ability to carry and disperse a range of pigments, wet low surface energy substrates, enhance paintability and drying, and increase adhesion to substrates. Organic solvents, such as alcohols, glycols, and glycol ethers, can help regulate drying speed, control foam, and reduce surface tension. Additives can act as adhesion promoters or binders, especially if pigment color adversely affects the adhesion and bonding properties of the formulation. All of these formulation variables affect the surface tension, and the coating will wet the substrate only when the surface tension is below the wetting tension, or critical surface energy, of the substrate. This does not guarantee that the ink will adhere to the substrate it wets, but verifying that this requirement is met is certainly a critical step in the right direction.
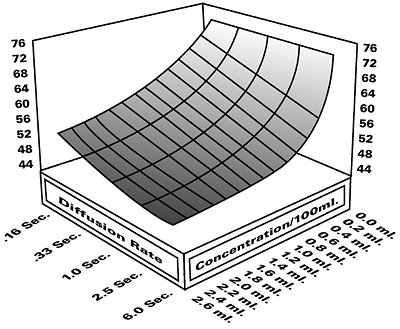
Figure 2 / Three-dimensional fluid properties
Surfactant properties
Ink formulators can measure and characterize the overall performance of surfactants in three dimensions; with respect to surface tension, concentration and diffusion time (surface age). This can be done manually, or a series of dynamic surface tension runs at increasing surfactant concentrations can be performed using a SensaDyne maximum bubble pressure tensiometer and an automated dispensing system. The result is a three-dimensional plot, as shown in Figure 2. The technique can also be used with surfactant-containing ink formulations to see the effect of formulation changes. Inactive additives primarily shift the curve up or down, while changes in surfactant type or concentration slope and/or shift the curve.
Slow diffusing surfactants may not sufficiently reduce surface tension to acceptable levels within dynamic coating time constraints and may partially cause defects such as: craters (bowl-shaped depressions) or pinholes; crawling or retraction (application dewetting of coatings); floating (mottled, spotted or streaked appearance); orange peel (uneven surface); and picture frame (edge build-up). Both rheology and surface tension modification are used to reduce or eliminate surface defects. For example, increasing viscosity can eliminate shrinkage, but if increasing viscosity is not feasible, the formulator may need to reduce the defect through surfactant modification.
Rapidly diffusing surfactants can alleviate surface defects by eliminating surface tension gradients. This occurs through rapid migration of surfactants from high concentrations (low surface tension) to low concentrations (high surface tension). Formulators sometimes mistakenly increase surfactant concentrations to reduce gradients instead of using better surfactants. This leads to higher surfactant costs and other problems. Surfactants that have both low equilibrium and dynamic surface tension in water do not necessarily have the same properties in highly formulated systems. Additional surfactants may become "tightly bound" to the polymer binder, or become solubilized by the pigment micelles, preventing further surface tension reduction.
Certain classes of surfactants have rapid diffusion properties due to their unique polar molecular structure. This type of surfactant lowers surface tension and has the added benefit of reducing or preventing foam. The hydrophobe-hydrophile-hydrophobe structure replaces materials that form a solid structural film at the interface and can make a good wetting agent or surface tension reducer. These acetylenic diol-based surfactants have highly branched alkyl groups and adsorb horizontally rather than vertically at the air/liquid interface. At low concentrations, these molecules cover large areas, but as concentrations increase they can be squeezed together, exhibiting unique compressible properties. The centrally located hydrophilic body gives the molecules a flat surface orientation, while the hydrophobic chain groups minimize the intermolecular attraction, which contributes to the low foam/defoam capability. A disadvantage of surfactants that only lower surface tension is that a separate defoamer may need to be added, which itself can lead to surface defects. Formulators should attempt to use a suitable surfactant that desirably has a low equilibrium and low dynamic surface tension value—low enough that the coating is applied to the substrate at the process speed at the desired viscosity. It is desirable if the surfactant used can perform more than one single function. Formulators should attempt to use a suitable surfactant that desirably has a low equilibrium and low dynamic surface tension value—low enough that the coating is applied to the substrate at the process speed at the desired viscosity. It is desirable if the surfactant used can perform more than one single function. Formulators should attempt to use a suitable surfactant that desirably has a low equilibrium and low dynamic surface tension value—low enough that the coating is applied to the substrate at the process speed at the desired viscosity. It is desirable if the surfactant used can perform more than one single function.
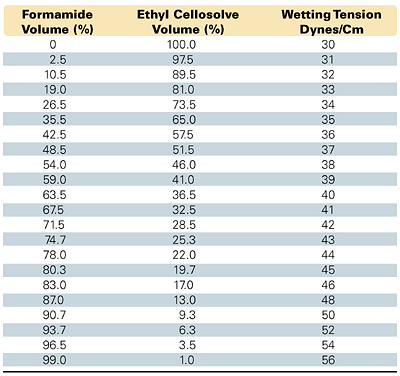
Figure 3 / Mixture of solutions for measuring wetting tension
surface energy
The transfer and spreading of the ink on the substrate depends on the surface energy of the material delivering the ink, the surface tension of the ink and the surface energy of the substrate receiving the ink. The substrate needs to have a higher surface energy than the ink and be attractive enough to facilitate good transfer and spreading, which in turn promotes good adhesion. Other factors that affect adhesion include the release characteristics of the surface, the composition of the substrate, and the structure of the substrate layer.
Measuring the wetting tension or surface energy of a substrate using a "dyne liquid" solution is common and inexpensive. These are purchased as a series of premixed solutions, each with a given wetting tension in dynes/cm. Users can also prepare their own solutions using a set of reagents such as formamide (HCONH2) and Union Carbide's ethyl Cellosolve® [ethylene glycol monoethyl ether (CH3CH2OCH2CH2OH)]. These specific non-reactive wetting solutions are prepared by mixing the two in the proportions specified in ASTM D 2578-84. This allows formulation of a range of wetting solutions according to ratios ranging from 30 to 56 dynes/cm, as shown in Figure 3. To make droplets or films more discernible, ASTM recommends adding small amounts of highly pigmented dyes.
The Dyne Liquid Solution (DLS) needs to wet the surface readily so that the wetting tension of the substrate is equal to the surface tension of the DLS used. The ASTM method clarifies it to a continuous 1 inch square film that remains intact for at least two seconds. If the liquid film spreads easily, a higher surface tension DLS is required. A lower surface tension DLS is required if the DLS breaks down into droplets (beading). The user is given an equivalent surface energy value for the substrate when the DLS remains spread out but has no particular tendency to bead up or spread further. The ASTM standard recommends working upwards, towards higher surface tensions, since wetting is easier to see than non-wetting. Problems with DLS formulations, whether purchased or mixed, can arise when the reagents are not used immediately because they have a limited "shelf life". Surface tension changes due to contamination and/or evaporation. For example, ethyl cellosolve is more volatile than formamide. When using these two agents, low dyne solutions containing a larger percentage of ethyl cellosolve evaporate more easily and lead to increased surface tension, a problem that has been demonstrated in long-term studies of wetting tension solutions.
The Max Bubble Pressure tensiometer validates purchased DLS formulations and simplifies two-reagent methods. Substrates were tested sequentially starting with a test solution of 100% ethyl cellosolve (30 dyne/cm) and gradually adding formamide to increase the surface tension of the test solution. Surface tension was measured prior to each progressive test, or when the final mixture was judged to sufficiently wet the substrate. This final surface tension value is equal to the wetting tension of the substrate. This applies to surface tensions up to 40 dynes/cm, where the ratio ends up being about two-thirds formamide and one-third ethyl cellosolve. If the substrate wetting tension is suspected to be higher than 37 dynes/cm, it will be faster to start with a one-to-one ratio.
This method eliminates the need for additional, often expensive and complex contact angle Measurement Instruments to determine the wetting tension. Another advantage of using a SensaDyne tensiometer is that, since temperature is measured together with surface tension, wetting tension can be determined under ambient conditions (e.g. on a factory floor) without the need for an environmental Test Chamber. For substrates that are likely to be tested at ambient temperature and humidity, the surface tension reading from the DLS gives the surface energy of the environment (wetting tension is most relevant) under conditions where the printing process is likely to occur.
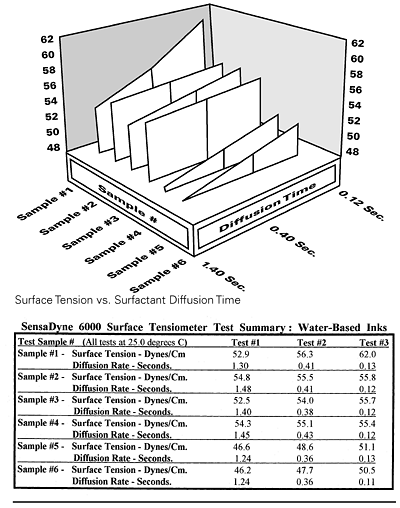
Figure 4 / Water-based ink
summarize
Instruments located near the formulation or press site need to be easy to use, suitable for both dynamic and static surface tension measurements, and immune to contamination and any adverse site conditions.
SensaDyne's dynamic surface tension measurement technology is based on a patented modification of the maximum bubble pressure method. Insert two probes with 4.0 mm and 0.5 mm holes into the fluid. A computerized instrument measures the maximum bubble pressure difference at the orifice and calculates the fluid surface tension. Air bubbles inside the fluid make the system immune to surface contamination and surface foam. The instrument is calibrated by pre-testing two fluid standards of known surface tension values, such as deionized water and alcohol. Immersing the probe in the middle of the sample requires user expertise.
Surface tension values are used to compare the quality of freshly formulated inks to previously measured inks. DLS wetting tension is used to determine and compare new substrates to previously used substrates. When the concentration of surfactants or other additives is varied, information can be obtained about the three-dimensional characterization of formulations containing active surfactants. The dynamic surface tension curves of a series of inks with active surfactants are shown in Fig. 4. Recipes exhibiting both low equilibrium and low dynamic values can be identified prior to use.
Comparing the data and dynamic curves for the first three water-based inks (samples #1 to #3) in Figure 4, it is found that sample #1 has a rather steep dynamic curve which should yield the lowest equilibrium surface tension once the curve flattens (to the left side) and reaches its minimum, at some point slower than the tested 1.4 seconds. However, it has the highest dynamic value (62) of the three at a surface age of 0.12 seconds. Sample #2 has the smallest slope and has the lowest dynamic value if the allowed diffusion time is much less than 0.12 seconds.

Figure 5 / Fountain Solution
However, Sample #3 had the lowest equilibrium (52.5) and dynamic (55.7) surface tension values of the tested surface ages. All other factors being equal, it may be the appropriate choice of the three. Comparing the last three formulations (Samples #4 to #6) in Figure 4 shows that Sample #4 is a suitable candidate for any process where surface aging is limited to 0.12 seconds or less. A similar analysis can be performed on a series of fountain solutions, as shown in Figure 5.
Formulation and coating issues can be greatly mitigated or completely avoided by measuring the surface tension and wetting tension of the supporting substrate prior to application of the ink. Mitigate problems faster and more aggressively by verifying and quantifying changes immediately after recipe adjustments. Formulators can improve ink transfer, spreading and adhesion by selecting surfactant and additive combinations that provide the right surface tension profile for the application.









