In addition to the basic components (salt and reducing agent of the metal to be plated), the actual chemical plating solution also contains other substances. Usually the situation is as follows:
1. Ligands that form soluble complexes with metal ions are necessary for alkaline solutions. In addition, the use of stable complexes sometimes enhances the autocatalytic effect.
2. Use substances that control and maintain a certain pH of the solution: the addition of buffers is particularly important because hydrogen ions are formed during the metal reduction process.
3. Stabilizers that slow down the reduction reaction in the bulk of the solution and thus enhance autocatalysis can be used. Sometimes, reagents such as whitening agents are also added to the solution.
deposition rate
The deposition rate is usually expressed in microns/hour (μm/h; or mil/h, μin./h, mg/cm2h). During deposition, if the concentration of the reacting species is not maintained at a constant level, the rate will decrease. The values given in the literature are usually averages and reflect only the initial phase.
This average rate depends on the ratio of the surface to be plated to the solution volume (dm2/l).
For the general case, the dependence of the deposition rate (v) on the reactant species concentration is quite complex. It is usually described by empirical equations such as:
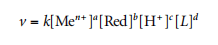
where k is the rate constant (constant value for a given type of system) and [L] is the concentration of free ligand (not bound to the metal ion in the complex). The exponents a and b are usually less than 1, where c is negative (using OH ion concentration in alkaline solutions, in which case the exponents are usually positive, O < c < 1). The exponent d is usually close to zero; however, when ligands are substituted, the deposition rate may change significantly. With constant concentrations of other solution components, the deposition rate decreases when the stability of the metal complex increases (as the concentration of free metal ions decreases); however, this relationship is not critical for the general case.
The electroless deposition rate of most metals under suitable conditions is about 2-5 μm/h, only the electroless nickel plating rate may be as high as 20 μm/h (this corresponds to an electroplating process with a current density of 200 A/m2)
Solution life
The solution lifetime represents the maximum duration that a solution is useful. The onset of metal ion reduction in the bulk of the solution may terminate its development. However, in most modern electroless plating solutions, volume reduction does not usually occur under normal operating conditions, and solution lifetime is limited by the accumulation of reaction products or impurities. Therefore, it is recommended not to characterize the life of the solution by time, which depends on the intensity of mining, but by the maximum amount of metal deposited or the number of turnovers from the volume unit of the solution (g/l or μm/l) to represent the initial amount of metal in the solution The number of possible deposits in the form of coatings. This number can be as large as 10 to 20. After the bad substances accumulated in the solution are removed, it can be used for a longer period of time, just like the electrolyte for electroplating.
After long-term use of the solution, a certain amount of precipitate may appear, because even in a completely stable solution, bulk reactions may proceed to a limited extent.
Reductant Efficiency Factor
Amount (in moles or grams) of reducing agent used to deposit one mole or gram
The concentration of the coating is represented by the reducing agent efficiency factor. In the actual electroless plating process, the required amount of reducing agent is exceeded (according to the reduction reaction), for example, equal to 2 moles for 1 mole of metal (nickel ion by hypophosphite reduction or copper ion by formaldehyde reduction) due to side reactions.
Solution Sensitivity to Activation
The minimum amount of catalyst that needs to be present on the dielectric surface to initiate the reduction reaction is shown by the sensitivity of the solution to activation. This parameter is related to solution stability. The lower the stability of the solution, the easier the reaction is to initiate, even on catalytically active surfaces. The high sensitivity of the solution to activation is not always desirable, since metals from such solutions can be deposited even on non-activated surfaces; in this case, selective plating becomes impossible. When palladium compounds are used for activation, there should be no plating for nickel and copper, respectively. When silver is used as an activator, it is only suitable for some electroless copper plating solutions, which require about 0.4 micrograms of silver per square centimeter.
