Electrolysis is a mixture of some of the better chromium salts and a selection of proprietary catalysts and additives to create its unique proprietary functionality. Carefully monitor the solution and maintain its quality through the use of strict, sophisticated equipment and laboratory controls. These materials are distributed only to recognized licensees in the Electrolizing Licensee Program.
thickness
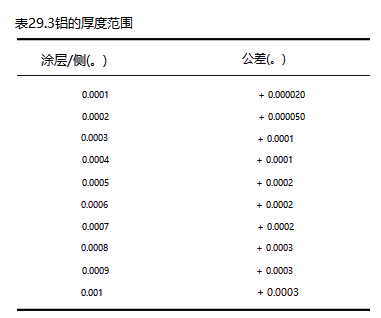
Electrolysis is performed in very thin, dense layers on the base metal. The electrolysis process doesn't build up on corners or sharp edges; it conforms perfectly to the surface. There is no change in the conductivity or magnetism of the base metal. Electrolytic deposits range from 0.000010 to 0.003 inches per side. Useful coatings range from 0.000025 to 0.01 inches. Average deposit is 0.0004 to 0.0008 inches per surface. Thickness tolerances of ±0.000010 to ±0.000050 inches can be maintained, depending on the thickness and quality level specified for that part. Coating thicknesses that may exceed 0.001 inch can be applied and electrical grinding operations can be maintained after tolerance elimination. Tables 29.2 and 29.3 list thickness ranges and tolerances for most metals.

Electrolytic thickness is always directly related to the base metal and will vary from one base metal to another. However, the thickness established for each will remain constant and predictable. In all applications where careful inspection is required to monitor the thickness of the deposit, Electrolizing, Inc. recommends the precise back etch method for determining the thickness of the deposited layer. This method is non-destructive to the base metal. If necessary, destructive photomicrographs will verify deposit thickness and consistency.
Adhesion
Electrolytic has excellent adhesive properties. The adhesion of the coating is such that when examined at four diameters, the bent specimen will not show separation from the base metal repeatedly through an angle of 180°, at diameters equal to the thickness of the specimen, until fracture.
Electrolytic coatings form a durable bond with the base metal by penetrating the surface pores, but can be removed by licensed electrolytic plants without adversely affecting the base metal.
corrosion
Electrolysis is resistant to attack by most organic and inorganic compounds ( acids except sulfuric and hydrochloric). The electrolytic coating is usually more noble than the substrate; therefore, it prevents porosity, cracks and discontinuities and by providing a uniform structure and chemical composition. The porosity, hardness, and poor surface finish of the base metal will affect the corrosion resistance of the electrolytic; however, all base metals that are electrolytically coated will have enhanced corrosion resistance.
Samples can be subjected to standard ASTM B-117 and B-287 salt spray tests. The electrolysis process also complies with the following specifications: QQ–C–320, AMS–2406, Mil–C–23422, Mil–P–6871, ANP–39, and ND-1002176.
Wear resistance (surface hardness)
Electrolytic is one of the hardest chrome surfaces available, measuring 70 to 72 Rc when applied. "As applied" means the measurable hardness of an electroplated coating, as measured on the base metal to which it is applied. The base metal plays an important role in determining the wear resistance of the electrolytic surface. Generally, electrolysis increases the measurable hardness by 10 to 15 points, as shown in Table 29.4.
In all cases the electrolysis was 70 to 72 Rc. However, the base metal directly affects the measurable hardness that can be achieved. The harder the base metal, the higher the electrolytically measurable hardness. Surface hardness measurements shall be made using the Knoop or Vickers method with a 5 to 10 gram load applied to the diamond point.

High hardness values indicate good wear resistance, but there are other factors to consider such as coating surface texture, coating density, substrate cleanliness prior to coating application, adhesion interface energy between coating and substrate, Adhesion between the coating surface and the coating can be relative to the sliding material, the type of lubricant used, and the combination of the opposing materials.
增加密度会提高耐磨性,因为它会减少裂纹、夹杂物和空隙,从而降低腐蚀速度并提供更多的抗碎裂、剥落和磨损能力。
表面疲劳磨损——真正剥落所属的类别——也影响传统 QQ–C–320 镀铬的耐磨性。这里的应力集中是在电沉积过程中产生的,这是形成铬晶体时的一个整体现象。电解涂层——本质上是一种铬合金——实际上没有这些内应力集中,因此剥落的可能性很小。这是电解的机械冲击条件下的耐磨性优于大多数常规镀铬的原因之一。
电解涂层还具有较低的动摩擦系数,并且在耐磨性方面,它优于所有其他镀铬层(包括化学镀镍和碳化钨涂层),在三种不同的测试机器上进行测量:Taber、Falex、和LFW-1。
最后,电解涂层表现出极低的粘着磨损系数:钢对铜合金为 1.72 × 101-7,钢对石油钢为 1.19 × 10-7(10-8 代表有史以来的最终或更佳磨损系数测量)。
润滑性
电镀涂层的摩擦系数为 0.11。 电解也会产生动摩擦在 LFW-1 试验机上使用氟硅油进行单向滑动试验条件下的系数低至 0.045,而在 Falex 润滑剂试验机上使用不含添加剂的白色矿物油时的系数低至 0.069。
当涉及极端温度时,电解的低摩擦系数是无价的。
一致性
当应用于相对光滑的表面(12 至 32 RMS 或更精细)时,电解效果更佳。 低于 4 RMS,该过程可能会稍微影响零件的精细光洁度,需要进行后电气化操作。
几乎所有形状和配置的内外表面都可以均匀加工。 宽度小于 0.187 英寸、深度大于宽度的槽或凹槽以及直径小于 0.187 英寸的孔需要特殊的工程设计以确保涂层均匀。 Electrolizing, Inc. 建议对此类零件进行试运行,并与 Electrolizing 工程师就尺寸小于 0.187 英寸的情况进行特别讨论。
耐热性
The maximum recommended operating temperature for electrolytic coating is approximately 1600°F (710°C). Time at temperature should be reviewed with Electrolizing, Inc. prior to testing or specifying electrolysis. Generally, oxidation occurs around 1100°F (430°C), progresses to 1650°F (740°C), and then diffuses.
brightness
The electroplating layer is smooth, continuous, fine-grained, uniform in adhesion, thickness and appearance, free of bubbles, pits, nodules, pores and edge accumulation. Electrolysis is used as a final coating for parts and equipment. Electrolytic coatings are glossy through application. However, a satin (matte) finish can be achieved if specified.
hydrogen embrittlement
In conventional chromium plating processes, a harmful side effect occurs: hydrogen occlusion. Hydrogen penetrates into the base material and causes embrittlement of the metal part, with subsequent reduction in mechanical properties, especially fatigue strength. Therefore, most traditional chrome plating control documents specify a final 375°F bake to remove hydrogen.
The longer the plating cycle, the greater the possibility of hydrogen embrittlement. Embrittlement is also more likely to occur after pickling. Shot peening and/or liquid honing can be used to relieve embrittlement stresses.
Hydrogen embrittlement is extremely unlikely to occur with electrolytic treatment because electrolytic treatment avoids most of the causes of true hydrogen embrittlement. Electrolizing, Inc. does not include post-process baking in its process technology. However, if the customer requires a post process bake, Electrolizing, Inc. can include it as a standard procedure.
