Perovskite is a calcium titanium oxide mineral with the chemical formula CaTiO 3 . The terms "perovskite" and "perovskite structure" are often used as if they were interchangeable. In fact, a true perovskite (mineral) is composed of calcium, titanium and oxygen in the form of CaTiO3 , and a perovskite structure is any substance with the general form ABX3 and has the same crystal as perovskite (mineral) structure. In the perovskite structure, "A" and "B" are two cations of very different sizes, and X is an anion bound to both. Perovskites occur in contact carbonate skarns in Magnet Cove, Arkansas, in metamorphic limestone blocks extruded by Mount Vesuvius, in chlorite and talc schists in the Urals and Switzerland, and as alkaline and mafic Auxiliary minerals of igneous rocks.
Where do perovskite materials come from?
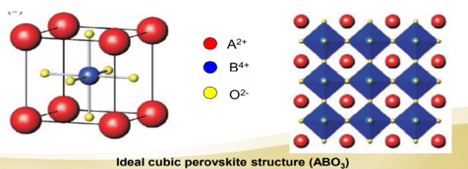
Perovskite is a perovskite oxide mineral composed of calcium titanate (CaTiO 3 ). The name "perovskite" is also applied to a class of compounds with the same crystal structure of CaTiO 3 ( XII A 2+VI B 4+ X 2- 3 ), known as the perovskite structure. Many different cations can be embedded in this structure, which enables the development of various engineered materials. Gustav Rose discovered perovskite in the Ural Mountains of Russia in 1839 and named it after Russian mineralogist LevPerovski (1792-1856).
How are perovskite materials made?
The perovskite structure has the general form ABX3, and any crystal structure of this form is assigned this name. True perovskite materials are made of oxygen, titanium and calcium in the form of CaTiO3 . However, the word perovskite also refers to a type of ceramic oxide with the molecular formula ABX. These compounds are known as alkali metal halide perovskites, inorganic oxide perovskites, and organometal halide perovskites. Bridgmanite, a silicate with the formula (Mg, Fe) SiO 3 , is a common mineral in the Earth's mantle. It employs a perovskite structure under high pressure. Compared with SiO 6 8− octahedral units, SiO 4 4− tetrahedral units in the dominant silica-containing minerals become unstable with increasing pressure. Under the pressure and temperature conditions of the lower mantle, the second most abundant element may be rock-salt periclase with a (Mg,Fe) oxide structure.

How do perovskite materials relate to quantum dot manufacturing?
Perovskite materials are a rising star in the photovoltaic industry. They are cheap to manufacture, easy to manufacture and very effective. Even better, they're new, and there's still a lot to be explored when it comes to more powerful solar cells. Perovskite materials are also used in LED technology. Quantum dots (QDs) are a very useful class of semiconductor materials with fascinating light-emitting properties, including the ability to adapt to any wavelength of light emitted by LEDs and solar cells.
A new class of quantum dots based on perovskite materials is currently being developed. Perovskite quantum dots are nanocrystalline semiconductors. They are more resistant to defects than metal chalcogenide quantum dots, and they have excellent photoluminescence quantum yields and high color purity that have equaled or exceeded metal chalcogenide quantum dots. These properties are excellent for electronic and optoelectronic applications, which means that perovskite quantum dots have great potential for real-world applications, including LED displays and solar cells with quantum dots.
Perovskite materials have received great attention from the research community due to their excellent photovoltaic performance. Recent studies have shown that reducing the size of the perovskite crystal structure to only a few nanometers can produce quantum dots with very high photoluminescence quantum yields and excellent color purity.
These quantum dots are very robust as they do not require any surface passivation* to maintain their high PLQY (the photoluminescence quantum yield of a molecule or material or PLQY is defined as the number of photons emitted as photons absorbed). In the case of defect and trap sites, their energy lies outside the bandgap, in the conduction or valence band. These nanocrystals of perovskite materials are easily synthesized in colloidal suspensions and easily integrated into optoelectronic devices using off-the-shelf processing techniques. This means that they have great application potential in future technologies.
Perovskite Quantum Dot Applications
Compared with other types of quantum dots, perovskite quantum dots have been less studied. However, to date, perovskite quantum dots have proven to be extremely effective in optoelectronics and nanotechnology, as well as in a variety of different applications. Perovskite quantum dots, for example, are used to make solar cells with power conversion efficiencies that exceed comparable devices based on more traditional nanocrystalline semiconductor materials.
Potential applications of perovskite quantum dots include:
X-ray image sensor
UV image sensor
led
LCD Monitor
Solar battery
single photon source
laser
PhotoDetector
quantum computing
cell imaging
Cancer Atlas



