Spectrophotometry uses Beer's law to quantitatively analyze the concentration of a substance in a solution based on its selective absorption of electromagnetic radiation at a specific wavelength. By measuring the intensity of light absorbed or transmitted by a sample, a Spectrophotometer is able to provide accurate qualitative and quantitative results. In the fields of chemistry, biochemistry, and environmental science, spectrophotometry is widely used in the determination of substance composition, quality control, and structure confirmation, and is one of the indispensable and important technical tools in modern analytical chemistry.
As a very important tool and technology in analytical chemistry, Spectrophotometer plays an irreplaceable role in modern scientific research and industrial production. The basic principle is to quantitatively analyze the concentration of chemical substances in a sample by comparing the difference in the intensity of light before and after the sample, taking advantage of the selective absorption properties of a substance to a specific wavelength of light.The working principle of a Spectrophotometer is based on the Beer-Lambert law, which states that the concentration of solute in a solution is directly proportional to how well the solution absorbs light at a particular wavelength. By selecting the appropriate wavelength and using a standard curve or reference solution, it is possible to accurately measure trace to large amounts of chemical components in a solution for qualitative and quantitative analysis.
Spectrophotometers have a wide range of applications in different fields. In environmental monitoring, it can be used to detect pollutants in water bodies, such as heavy metal ions and organic matter, so as to assess water quality; In food safety testing, it can track the nutrients or harmful additives in food; In pharmaceutical analysis, the content and purity of drugs can be determined to ensure the safety and efficacy of drugs.In addition, the application of Spectrophotometer s is not limited to liquid samples, but can also be extended to the analysis of solid samples and gaseous samples, enabling fast and accurate analysis of a wide range of complex samples with appropriate optical paths and sample preparation techniques.

1. Principles and basic concepts of spectrophotometry
1. Selective absorption characteristics
Selective absorption property refers to the specificity of a substance's ability to absorb light at a particular wavelength relative to other wavelengths. Each chemical has its own unique absorption spectrum, which is the ability to absorb light energy at a particular wavelength higher than other wavelengths. This selectivity allows Spectrophotometer s to accurately determine the concentration or amount of a particular chemical in a sample by measuring the difference in the intensity of light before and after the sample. By selecting the right wavelength, Spectrophotometer s enable simultaneous measurement of multiple chemical compositions and provide highly accurate results in different applications.
2. Beer's Law
Beer's law, also known as Beer-Lambert's law or Lambert-Beale law, is a law that describes the relationship between the concentration of solutes in a solution and the absorption of light by the solution. Beer's law states that the absorbance of a solution to a particular wavelength of light is directly proportional to its solute concentration given the length of the optical path length and molar absorbance coefficient. This relationship is the basis for spectrophotometric measurement of solute concentration in solution. By measuring the absorbance of the solution and the known molar absorbance coefficient, the concentration of solutes in the solution can be calculated, enabling quantitative analysis.
3. Wavelength and wavenumber
The ultraviolet spectral region (UV) covers the wavelength range from 10 nm to 400 nm. In analytical chemistry, ultraviolet spectroscopy is often used to study the electronic transition processes of molecules, especially π-π and n-π transitions. These transitions are ubiquitous in organic compounds, and UV spectroscopy provides valuable information about molecular structure and electronic state by measuring the absorption of a sample under UV light.
The visible spectral region is located between the ultraviolet and infrared spectrums, with wavelengths ranging from 400 nm to 700 nm. Visible light is the spectral range visible to the human eye and is also a spectral area commonly used in daily life and laboratories. In analytical chemistry, visible spectroscopy is widely used to determine the concentration of a substance in a solution, and to determine the degree of absorption or reflection of visible light in a solution by colorimetric or spectrophotometry, so as to infer the concentration or mass of a substance in a solution.
The infrared spectral region (IR) includes wavelengths ranging from 700 nm to 1 mm. Infrared spectroscopy is used to study molecular vibrations and rotation patterns in matter, which are closely related to the functional groups and structure of molecules. By measuring the absorption spectrum of a substance under infrared light, the molecular structure and chemical composition of a substance can be determined, which is widely used in organic chemistry, materials science, and biochemistry.

2. Composition and structure of Spectrophotometer
1. Light source
Different types of light sources have their own application characteristics and areas in scientific research and industrial applications. As a high-brightness, continuous spectrum light source, hydrogen arc lamp is mainly used in the fields of spectral analysis, fluorescence spectroscopy and chemical reaction kinetics. Its luminescence continuum covers a wide range of wavelengths, making it suitable for experiments that require high resolution and sensitivity. Tungsten lamps, on the other hand, are widely used in Spectrophotometer s, microscopes, spectrometers, and optical microscopes due to their stable light output and long life. Its spectral characteristics are close to those of natural light, especially suitable for occasions that require long-term stable operation and high brightness requirements. The choice of these light sources depends on the specific application needs, ensuring the best optical performance and data quality during experiments and applications.
2. Monochromator
Horizontal autocollimation systems, vertical autocollimation systems, and Zeny-Turner systems are common types of monochromators that play a key role in spectroscopy instrumentation. The horizontal autocollimation system achieves high resolution and high optical efficiency through a combination of grating and lens, making it suitable for applications that require precise wavelength selection and high resolution. The vertical autocollimation system provides higher spectral brightness and a larger wavelength range by mounting the grating vertically in the optical path, which is suitable for experiments that require higher spectral brightness and a larger wavelength range. The Zeny-Turner system combines the diffraction of the grating and the imaging function of the optical lens, with good optical performance and high spectral resolution, and is suitable for applications with high requirements for resolution and wavelength accuracy.
3. Sample room
The specimen chamber plays a key role in spectroscopy instrumentation in carrying the sample and interacting with light. Gas specimen chambers are typically hermetically sealed, allowing gas samples to be introduced into the optical path for analysis. They are usually equipped with gas inlet and outlet and pressure control devices to ensure accurate measurements under different pressure and environmental conditions, and are commonly used in the study of gas phase reactions, air pollution monitoring, etc. The liquid specimen chamber is designed as a container that can hold the liquid sample, usually made of optical grade glass or quartz to maintain high clarity and chemical inertness. This sample chamber is commonly used in solution analysis, medicinal chemistry, biochemistry, and other fields, and can provide high-precision absorption and reflectance spectral data, as well as the analysis and study of liquid samples. Whether gas or liquid specimen chambers, they are designed and applied to ensure efficient interaction between the sample and the light source to provide reliable spectral results.
4. Detection system
Photoelectric receivers work on the principle of the photoelectric effect, and when a photon hits its light-sensitive surface, an electric charge or current is generated. These receivers, typically made of semiconductor materials such as silicon or indium gallium germanium, are highly sensitive and responsive, making them suitable for spectral measurements from the ultraviolet to the near-infrared wavelength. Photoelectric receivers are widely used in molecular spectroscopy, chemical analysis, biomedicine and other fields, which can quickly and accurately convert optical signals into electrical signals, and then carry out data acquisition and analysis.
In contrast, a thermoelectric receiver uses the thermoelectric effect to detect an optical signal. When a beam of light hits its surface, the surface creates small temperature changes that are converted into a voltage signal by a thermoelectric element, usually a thermocouple or thermopile. Thermoelectric receivers are favored for their wide response to the spectral wavelength range and high linear response, especially for the measurement and analysis of infrared and far-infrared spectroscopy, such as combustion gas analysis, crystallography research in materials science, etc.
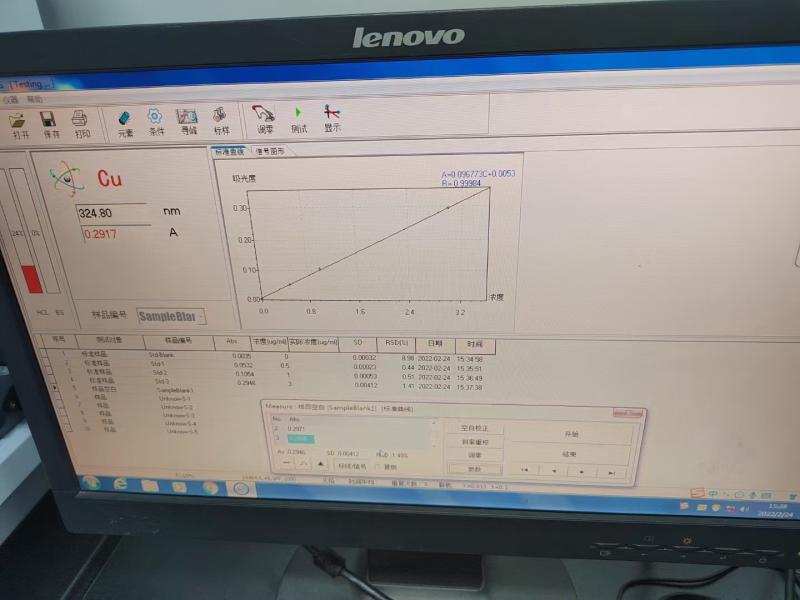
Third, the field of application
Spectrophotometers play a key role in several application areas. First, it enables accurate identification of substances and determination of their structure in qualitative analysis by means of absorption spectroscopy, a process based on the selective absorption of light at a specific wavelength by the sample. Secondly, in terms of quantitative analysis, Spectrophotometer s utilize Beer's law for concentration determination, a light-based measurement method that is not only fast but also highly accurate, so it is widely used in chemical analysis and environmental monitoring. Finally, due to its high sensitivity and precision, spectrophotometry is particularly suitable for the determination and quality control of trace components, such as food safety, drug manufacturing, and water quality testing, providing important support for ensuring product quality and safety.
Today we discussed several aspects and applications of Spectrophotometer s. The basic principle of spectrophotometry, the selective absorption of light at a specific wavelength by a sample, is widely used in qualitative analysis to help identify substances and determine their structure. We also discuss the importance of spectrophotometry in quantitative analysis, especially concentration determination through Beer's law, which is not only fast but also highly accurate, and is widely used in chemical analysis and environmental monitoring. In addition, we touched on the role of Spectrophotometer s in various application areas, in particular their advantages in high sensitivity and precision in microfraction determination and quality control. As an important analytical tool, Spectrophotometer provides necessary analytical support and technical support for many fields such as scientific research, industrial production and environmental protection.













